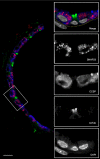
SNAP23 is selectively expressed in airway secretory cells and mediates baseline and stimulated mucin secretion
- PMID: 26182382
- PMCID: PMC4613665
- DOI: 10.1042/BSR20150004
SNAP23 is selectively expressed in airway secretory cells and mediates baseline and stimulated mucin secretion
Abstract
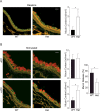
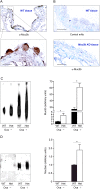
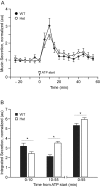
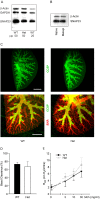
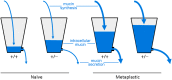
Airway mucin secretion is important pathophysiologically and as a model of polarized epithelial regulated exocytosis. We find the trafficking protein, SNAP23 (23-kDa paralogue of synaptosome-associated protein of 25 kDa), selectively expressed in secretory cells compared with ciliated and basal cells of airway epithelium by immunohistochemistry and FACS, suggesting that SNAP23 functions in regulated but not constitutive epithelial secretion. Heterozygous SNAP23 deletant mutant mice show spontaneous accumulation of intracellular mucin, indicating a defect in baseline secretion. However mucins are released from perfused tracheas of mutant and wild-type (WT) mice at the same rate, suggesting that increased intracellular stores balance reduced release efficiency to yield a fully compensated baseline steady state. In contrast, acute stimulated release of intracellular mucin from mutant mice is impaired whether measured by a static imaging assay 5 min after exposure to the secretagogue ATP or by kinetic analysis of mucins released from perfused tracheas during the first 10 min of ATP exposure. Together, these data indicate that increased intracellular stores cannot fully compensate for the defect in release efficiency during intense stimulation. The lungs of mutant mice develop normally and clear bacteria and instilled polystyrene beads comparable to WT mice, consistent with these functions depending on baseline secretion that is fully compensated.
Keywords: 23-kDa paralogue of synaptosome-associated protein of 25 kDa (SNAP23); exocytosis; mucin; mucus; secretion.
© 2015 The Author(s).
Figures