Single-Cell mRNA Profiling Reveals Cell-Type-Specific Expression of Neurexin Isoforms
- PMID: 26182417
- PMCID: PMC4733560
- DOI: 10.1016/j.neuron.2015.06.028
Single-Cell mRNA Profiling Reveals Cell-Type-Specific Expression of Neurexin Isoforms
Abstract
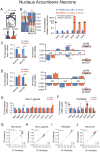
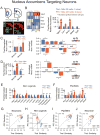
Neurexins are considered central organizers of synapse architecture that are implicated in neuropsychiatric disorders. Expression of neurexins in hundreds of alternatively spliced isoforms suggested that individual neurons might exhibit a cell-type-specific neurexin expression pattern (a neurexin code). To test this hypothesis, we quantified the single-cell levels of neurexin isoforms and other trans-synaptic cell-adhesion molecules by microfluidics-based RT-PCR. We show that the neurexin repertoire displays pronounced cell-type specificity that is remarkably consistent within each type of neuron. Furthermore, we uncovered region-specific regulation of neurexin transcription and splice-site usage. Finally, we demonstrate that the transcriptional profiles of neurexins can be altered in an experience-dependent fashion by exposure to a drug of abuse. Our data provide evidence of cell-type-specific expression patterns of multiple neurexins at the single-cell level and suggest that expression of synaptic cell-adhesion molecules overlaps with other key features of cellular identity and diversity.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures






References
-
- Araç D, Boucard AA, Ozkan E, Strop P, Newell E, Südhof TC, Brunger AT. Structures of neuroligin-1 and the neuroligin-1/neurexin-1 beta complex reveal specific protein-protein and protein-Ca2+ interactions. Neuron. 2007;56:992–1003. - PubMed
-
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Südhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

