Acute exposure to silica nanoparticles aggravate airway inflammation: different effects according to surface characteristics
- PMID: 26183169
- PMCID: PMC4525300
- DOI: 10.1038/emm.2015.50
Acute exposure to silica nanoparticles aggravate airway inflammation: different effects according to surface characteristics
Abstract
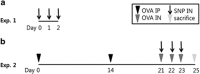
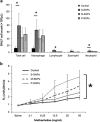
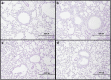
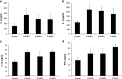
Silica nanoparticles (SNPs) are widely used in many scientific and industrial fields despite the lack of proper evaluation of their potential toxicity. This study examined the effects of acute exposure to SNPs, either alone or in conjunction with ovalbumin (OVA), by studying the respiratory systems in exposed mouse models. Three types of SNPs were used: spherical SNPs (S-SNPs), mesoporous SNPs (M-SNPs), and PEGylated SNPs (P-SNPs). In the acute SNP exposure model performed, 6-week-old BALB/c female mice were intranasally inoculated with SNPs for 3 consecutive days. In the OVA/SNPs asthma model, the mice were sensitized two times via the peritoneal route with OVA. Additionally, the mice endured OVA with or without SNP challenges intranasally. Acute SNP exposure induced significant airway inflammation and airway hyper-responsiveness, particularly in the S-SNP group. In OVA/SNPs asthma models, OVA with SNP-treated group showed significant airway inflammation, more than those treated with only OVA and without SNPs. In these models, the P-SNP group induced lower levels of inflammation on airways than both the S-SNP or M-SNP groups. Interleukin (IL)-5, IL-13, IL-1β and interferon-γ levels correlated with airway inflammation in the tested models, without statistical significance. In the mouse models studied, increased airway inflammation was associated with acute SNPs exposure, whether exposed solely to SNPs or SNPs in conjunction with OVA. P-SNPs appear to be relatively safer for clinical use than S-SNPs and M-SNPs, as determined by lower observed toxicity and airway system inflammation.
Figures



Similar articles
-
Toxic and adjuvant effects of silica nanoparticles on ovalbumin-induced allergic airway inflammation in mice.Respir Res. 2016 May 18;17(1):60. doi: 10.1186/s12931-016-0376-x. Respir Res. 2016. PMID: 27194244 Free PMC article.
-
Adjuvant effects of gaseous formaldehyde on the hyper-responsiveness and inflammation in a mouse asthma model immunized by ovalbumin.J Immunotoxicol. 2011 Oct-Dec;8(4):305-14. doi: 10.3109/1547691X.2011.600738. Epub 2011 Aug 19. J Immunotoxicol. 2011. PMID: 21854218
-
Spleen tyrosine kinase inhibition attenuates airway hyperresponsiveness and pollution-induced enhanced airway response in a chronic mouse model of asthma.J Allergy Clin Immunol. 2013 Feb;131(2):512-20.e1-10. doi: 10.1016/j.jaci.2012.07.039. Epub 2012 Sep 13. J Allergy Clin Immunol. 2013. PMID: 22981792
-
Copper oxide nanoparticles aggravate airway inflammation and mucus production in asthmatic mice via MAPK signaling.Nanotoxicology. 2016;10(4):445-52. doi: 10.3109/17435390.2015.1078851. Epub 2015 Oct 15. Nanotoxicology. 2016. PMID: 26472121
-
Silica dioxide nanoparticles aggravate airway inflammation in an asthmatic mouse model via NLRP3 inflammasome activation.Regul Toxicol Pharmacol. 2020 Apr;112:104618. doi: 10.1016/j.yrtph.2020.104618. Epub 2020 Feb 19. Regul Toxicol Pharmacol. 2020. PMID: 32087352
Cited by
-
Empagliflozin and Dulaglutide are Effective against Obesity-induced Airway Hyperresponsiveness and Fibrosis in A Murine Model.Sci Rep. 2019 Oct 30;9(1):15601. doi: 10.1038/s41598-019-51648-1. Sci Rep. 2019. PMID: 31666643 Free PMC article.
-
Activation of MAPK and Cyclin D1/CDK4 in Malignant Transformation of Human Embryonic Lung Fibroblasts Induced by Silica and Benzopyrene.Asian Pac J Cancer Prev. 2020 Feb 1;21(2):295-300. doi: 10.31557/APJCP.2020.21.2.295. Asian Pac J Cancer Prev. 2020. PMID: 32102502 Free PMC article.
-
Nutritional and Nanotechnological Modulators of Microglia.Front Immunol. 2016 Jul 15;7:270. doi: 10.3389/fimmu.2016.00270. eCollection 2016. Front Immunol. 2016. PMID: 27471505 Free PMC article. Review.
-
Magnetic Mesoporous Silica Nanorods Loaded with Ceria and Functionalized with Fluorophores for Multimodal Imaging.ACS Appl Nano Mater. 2022 Feb 25;5(2):2113-2125. doi: 10.1021/acsanm.1c03837. Epub 2022 Feb 10. ACS Appl Nano Mater. 2022. PMID: 35252779 Free PMC article.
-
Surface PEGylation suppresses pulmonary effects of CuO in allergen-induced lung inflammation.Part Fibre Toxicol. 2019 Jul 5;16(1):28. doi: 10.1186/s12989-019-0309-1. Part Fibre Toxicol. 2019. PMID: 31277695 Free PMC article.
References
-
- Roco MC. Nanotechnology: convergence with modern biology and medicine. Curr Opin Biotechnol. 2003;14:337–346. - PubMed
-
- Vallet-Regi M, Balas F, Arcos D. Mesoporous materials for drug delivery. Angew Chem Int Ed Engl. 2007;46:7548–7558. - PubMed
-
- Carino IS, Pasqua L, Testa F, Aiello R, Puoci F, Iemma F, et al. Silica-based mesoporous materials as drug delivery system for methotrexate release. Drug Deliv. 2007;14:491–495. - PubMed
-
- Maynard AD. Nanotechnology: the next big thing, or much ado about nothing. Ann Occup Hyg. 2007;51:1–12. - PubMed
-
- Nemmar A, Al-Salam S, Zia S, Yasin J, Al Husseni I, Ali BH. Diesel exhaust particles in the lung aggravate experimental acute renal failure. Toxicol Sci. 2010;113:267–277. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

