A standardized and reproducible protocol for serum-free monolayer culturing of primary paediatric brain tumours to be utilized for therapeutic assays
- PMID: 26183281
- PMCID: PMC4505308
- DOI: 10.1038/srep12218
A standardized and reproducible protocol for serum-free monolayer culturing of primary paediatric brain tumours to be utilized for therapeutic assays
Abstract
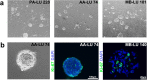
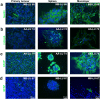
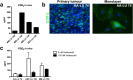
In vitro cultured brain tumour cells are indispensable tools for drug screening and therapeutic development. Serum-free culture conditions tentatively preserve the features of the original tumour, but commonly comprise neurosphere propagation, which is a technically challenging procedure. Here, we define a simple, non-expensive and reproducible serum-free cell culture protocol for establishment and propagation of primary paediatric brain tumour cultures as adherent monolayers. The success rates for establishment of primary cultures (including medulloblastomas, atypical rhabdoid tumour, ependymomas and astrocytomas) were 65% (11/17) and 78% (14/18) for sphere cultures and monolayers respectively. Monolayer culturing was particularly feasible for less aggressive tumour subsets, where neurosphere cultures could not be generated. We show by immunofluorescent labelling that monolayers display phenotypic similarities with corresponding sphere cultures and primary tumours, and secrete clinically relevant inflammatory factors, including PGE2, VEGF, IL-6, IL-8 and IL-15. Moreover, secretion of PGE2 was considerably reduced by treatment with the COX-2 inhibitor Valdecoxib, demonstrating the functional utility of our newly established monolayer for preclinical therapeutic assays. Our findings suggest that this culture method could increase the availability and comparability of clinically representative in vitro models of paediatric brain tumours, and encourages further molecular evaluation of serum-free monolayer cultures.
Figures


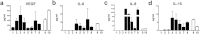
Similar articles
-
Identification of anti-tumour biologics using primary tumour models, 3-D phenotypic screening and image-based multi-parametric profiling.Mol Cancer. 2015 Jul 31;14:147. doi: 10.1186/s12943-015-0415-0. Mol Cancer. 2015. PMID: 26227951 Free PMC article.
-
Culture methods of diffuse intrinsic pontine glioma cells determine response to targeted therapies.Exp Cell Res. 2017 Nov 15;360(2):397-403. doi: 10.1016/j.yexcr.2017.09.032. Epub 2017 Sep 22. Exp Cell Res. 2017. PMID: 28947132
-
An efficient method for derivation and propagation of glioblastoma cell lines that conserves the molecular profile of their original tumours.J Neurosci Methods. 2009 Jan 30;176(2):192-9. doi: 10.1016/j.jneumeth.2008.07.022. J Neurosci Methods. 2009. PMID: 19215724
-
The use of 3-D cultures for high-throughput screening: the multicellular spheroid model.J Biomol Screen. 2004 Jun;9(4):273-85. doi: 10.1177/1087057104265040. J Biomol Screen. 2004. PMID: 15191644 Review.
-
Experimental anti-tumor therapy in 3-D: spheroids--old hat or new challenge?Int J Radiat Biol. 2007 Nov-Dec;83(11-12):849-71. doi: 10.1080/09553000701727531. Int J Radiat Biol. 2007. PMID: 18058370 Review.
Cited by
-
Characterization of EGF-guided MDA-MB-231 cell chemotaxis in vitro using a physiological and highly sensitive assay system.PLoS One. 2018 Sep 13;13(9):e0203040. doi: 10.1371/journal.pone.0203040. eCollection 2018. PLoS One. 2018. PMID: 30212492 Free PMC article.
-
Scalable Production of Glioblastoma Tumor-initiating Cells in 3 Dimension Thermoreversible Hydrogels.Sci Rep. 2016 Aug 23;6:31915. doi: 10.1038/srep31915. Sci Rep. 2016. PMID: 27549983 Free PMC article.
-
Inter and intra-tumor heterogeneity of paediatric type diffuse high-grade gliomas revealed by single-cell mass cytometry.Front Oncol. 2022 Dec 8;12:1016343. doi: 10.3389/fonc.2022.1016343. eCollection 2022. Front Oncol. 2022. PMID: 36568177 Free PMC article.
-
Conditional reprogramming culture conditions facilitate growth of lower-grade glioma models.Neuro Oncol. 2021 May 5;23(5):770-782. doi: 10.1093/neuonc/noaa263. Neuro Oncol. 2021. PMID: 33258947 Free PMC article.
-
Pediatric low-grade glioma models: advances and ongoing challenges.Front Oncol. 2024 Jan 22;13:1346949. doi: 10.3389/fonc.2023.1346949. eCollection 2023. Front Oncol. 2024. PMID: 38318325 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

