Fibroblast Growth Factor 9 Imparts Hierarchy and Vasoreactivity to the Microcirculation of Renal Tumors and Suppresses Metastases
- PMID: 26183774
- PMCID: PMC4571963
- DOI: 10.1074/jbc.M115.652222
Fibroblast Growth Factor 9 Imparts Hierarchy and Vasoreactivity to the Microcirculation of Renal Tumors and Suppresses Metastases
Abstract
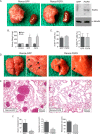
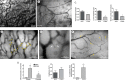
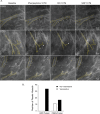
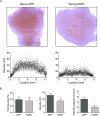
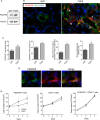
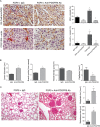
Tumor vessel normalization has been proposed as a therapeutic paradigm. However, normal microvessels are hierarchical and vasoreactive with single file transit of red blood cells through capillaries. Such a network has not been identified in malignant tumors. We tested whether the chaotic tumor microcirculation could be reconfigured by the mesenchyme-selective growth factor, FGF9. Delivery of FGF9 to renal tumors in mice yielded microvessels that were covered by pericytes, smooth muscle cells, and a collagen-fortified basement membrane. This was associated with reduced pulmonary metastases. Intravital microvascular imaging revealed a haphazard web of channels in control tumors but a network of arterioles, bona fide capillaries, and venules in FGF9-expressing tumors. Moreover, whereas vasoreactivity was absent in control tumors, arterioles in FGF9-expressing tumors could constrict and dilate in response to adrenergic and nitric oxide releasing agents, respectively. These changes were accompanied by reduced hypoxia in the tumor core and reduced expression of the angiogenic factor VEGF-A. FGF9 was found to selectively amplify a population of PDGFRβ-positive stromal cells in the tumor and blocking PDGFRβ prevented microvascular differentiation by FGF9 and also worsened metastases. We conclude that harnessing local mesenchymal stromal cells with FGF9 can differentiate the tumor microvasculature to an extent not observed previously.
Keywords: angiogenesis; fibroblast growth factor (FGF); hypoxia; metastasis; microvascular flow; tumor microenvironment; vascular biology; vasoreactivity.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
FGF9 and SHH regulate mesenchymal Vegfa expression and development of the pulmonary capillary network.Development. 2007 Oct;134(20):3743-52. doi: 10.1242/dev.004879. Epub 2007 Sep 19. Development. 2007. PMID: 17881491 Free PMC article.
-
FGF9-induced ovarian cancer cell invasion involves VEGF-A/VEGFR2 augmentation by virtue of ETS1 upregulation and metabolic reprogramming.J Cell Biochem. 2018 Nov;119(10):8174-8189. doi: 10.1002/jcb.26820. Epub 2018 Jun 15. J Cell Biochem. 2018. PMID: 29904943
-
Effect of FGF/FGFR pathway blocking on lung adenocarcinoma and its cancer-associated fibroblasts.J Pathol. 2019 Oct;249(2):193-205. doi: 10.1002/path.5290. Epub 2019 Jun 24. J Pathol. 2019. PMID: 31090071
-
Microvascular dysfunction and kidney disease: Challenges and opportunities?Microcirculation. 2021 Apr;28(3):e12661. doi: 10.1111/micc.12661. Epub 2020 Oct 28. Microcirculation. 2021. PMID: 33025626 Free PMC article. Review.
-
Formation of microvascular networks: role of stromal interactions directing angiogenic growth.Microcirculation. 2014 May;21(4):278-89. doi: 10.1111/micc.12115. Microcirculation. 2014. PMID: 24447042 Free PMC article. Review.
Cited by
-
Differentiation of arterioles from venules in mouse histology images using machine learning.J Med Imaging (Bellingham). 2017 Apr;4(2):021104. doi: 10.1117/1.JMI.4.2.021104. Epub 2017 Feb 28. J Med Imaging (Bellingham). 2017. PMID: 28331891 Free PMC article.
-
Intussusceptive angiogenesis-on-a-chip: Evidence for transluminal vascular bridging by endothelial delamination.Proc Natl Acad Sci U S A. 2025 Apr 22;122(16):e2423700122. doi: 10.1073/pnas.2423700122. Epub 2025 Apr 17. Proc Natl Acad Sci U S A. 2025. PMID: 40244661 Free PMC article.
-
DJ-1 promotes epithelial-to-mesenchymal transition via enhancing FGF9 expression in colorectal cancer.Biol Open. 2020 May 19;9(5):bio051680. doi: 10.1242/bio.051680. Biol Open. 2020. PMID: 32366371 Free PMC article.
-
Low-flow intussusception and metastable VEGFR2 signaling launch angiogenesis in ischemic muscle.Sci Adv. 2021 Nov 26;7(48):eabg9509. doi: 10.1126/sciadv.abg9509. Epub 2021 Nov 26. Sci Adv. 2021. PMID: 34826235 Free PMC article.
-
Delayed Tooth Development and the Impaired Differentiation of Stem/Progenitor Cells in Incisors from Type 2 Diabetes Mice.Int J Mol Sci. 2024 Dec 19;25(24):13619. doi: 10.3390/ijms252413619. Int J Mol Sci. 2024. PMID: 39769381 Free PMC article.
References
-
- Folkman J. (1971) Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 - PubMed
-
- Chung A. S., Lee J., Ferrara N. (2010) Targeting the tumour vasculature: insights from physiological angiogenesis. Nat. Rev. Cancer 10, 505–514 - PubMed
-
- Jain R. K. (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

