Accumulation of Mitochondrial DNA Mutations Disrupts Cardiac Progenitor Cell Function and Reduces Survival
- PMID: 26183775
- PMCID: PMC4571958
- DOI: 10.1074/jbc.M115.649657
Accumulation of Mitochondrial DNA Mutations Disrupts Cardiac Progenitor Cell Function and Reduces Survival
Erratum in
-
Accumulation of mitochondrial DNA mutations disrupts cardiac progenitor cell function and reduces survival.J Biol Chem. 2017 Jul 7;292(27):11348. doi: 10.1074/jbc.A115.649657. J Biol Chem. 2017. PMID: 28687597 Free PMC article. No abstract available.
Abstract
Transfer of cardiac progenitor cells (CPCs) improves cardiac function in heart failure patients. However, CPC function is reduced with age, limiting their regenerative potential. Aging is associated with numerous changes in cells including accumulation of mitochondrial DNA (mtDNA) mutations, but it is unknown how this impacts CPC function. Here, we demonstrate that acquisition of mtDNA mutations disrupts mitochondrial function, enhances mitophagy, and reduces the replicative and regenerative capacities of the CPCs. We show that activation of differentiation in CPCs is associated with expansion of the mitochondrial network and increased mitochondrial oxidative phosphorylation. Interestingly, mutant CPCs are deficient in mitochondrial respiration and rely on glycolysis for energy. In response to differentiation, these cells fail to activate mitochondrial respiration. This inability to meet the increased energy demand leads to activation of cell death. These findings demonstrate the consequences of accumulating mtDNA mutations and the importance of mtDNA integrity in CPC homeostasis and regenerative potential.
Keywords: aging; cardiac progenitor cells; glycolysis; heart failure; mitochondria; mitochondrial DNA (mtDNA); mitophagy; stem cells.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

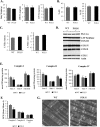

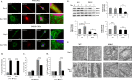

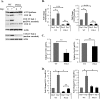
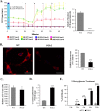

Similar articles
-
BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation.Autophagy. 2019 Jul;15(7):1182-1198. doi: 10.1080/15548627.2019.1580095. Epub 2019 Feb 22. Autophagy. 2019. PMID: 30741592 Free PMC article.
-
Somatic cells with a heavy mitochondrial DNA mutational load render induced pluripotent stem cells with distinct differentiation defects.Stem Cells. 2014 May;32(5):1173-82. doi: 10.1002/stem.1630. Stem Cells. 2014. PMID: 24446123
-
Exercise-induced mitochondrial p53 repairs mtDNA mutations in mutator mice.Skelet Muscle. 2016 Jan 31;6:7. doi: 10.1186/s13395-016-0075-9. eCollection 2016. Skelet Muscle. 2016. Retraction in: Skelet Muscle. 2021 Mar 30;11(1):8. doi: 10.1186/s13395-021-00264-7. PMID: 26834962 Free PMC article. Retracted.
-
Mitochondria: Biogenesis and mitophagy balance in segregation and clonal expansion of mitochondrial DNA mutations.Int J Biochem Cell Biol. 2015 Jun;63:21-4. doi: 10.1016/j.biocel.2015.01.023. Epub 2015 Feb 7. Int J Biochem Cell Biol. 2015. PMID: 25666555 Review.
-
Respiratory function decline and DNA mutation in mitochondria, oxidative stress and altered gene expression during aging.Chang Gung Med J. 2009 Mar-Apr;32(2):113-32. Chang Gung Med J. 2009. PMID: 19403001 Review.
Cited by
-
Endoplasmic reticulum stress and alterations of peroxiredoxins in aged hearts.Mech Ageing Dev. 2023 Oct;215:111859. doi: 10.1016/j.mad.2023.111859. Epub 2023 Sep 1. Mech Ageing Dev. 2023. PMID: 37661065 Free PMC article.
-
BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation.Autophagy. 2019 Jul;15(7):1182-1198. doi: 10.1080/15548627.2019.1580095. Epub 2019 Feb 22. Autophagy. 2019. PMID: 30741592 Free PMC article.
-
Decline in cellular function of aged mouse c-kit+ cardiac progenitor cells.J Physiol. 2017 Oct 1;595(19):6249-6262. doi: 10.1113/JP274775. Epub 2017 Aug 18. J Physiol. 2017. PMID: 28737214 Free PMC article.
-
Roles of Mitochondrial DNA Mutations in Stem Cell Ageing.Genes (Basel). 2018 Mar 27;9(4):182. doi: 10.3390/genes9040182. Genes (Basel). 2018. PMID: 29584704 Free PMC article. Review.
-
Possible Role of Mitochondrial DNA Mutations in Chronification of Inflammation: Focus on Atherosclerosis.J Clin Med. 2020 Apr 1;9(4):978. doi: 10.3390/jcm9040978. J Clin Med. 2020. PMID: 32244740 Free PMC article. Review.
References
-
- Beltrami A. P., Barlucchi L., Torella D., Baker M., Limana F., Chimenti S., Kasahara H., Rota M., Musso E., Urbanek K., Leri A., Kajstura J., Nadal-Ginard B., Anversa P. (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 - PubMed
-
- Ellison G. M., Vicinanza C., Smith A. J., Aquila I., Leone A., Waring C. D., Henning B. J., Stirparo G. G., Papait R., Scarfò M., Agosti V., Viglietto G., Condorelli G., Indolfi C., Ottolenghi S., Torella D., Nadal-Ginard B. (2013) Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 154, 827–842 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HL113656/HL/NHLBI NIH HHS/United States
- T32GM007752/GM/NIGMS NIH HHS/United States
- R01 HL087023/HL/NHLBI NIH HHS/United States
- R01 HL122525/HL/NHLBI NIH HHS/United States
- F31HL123309/HL/NHLBI NIH HHS/United States
- R01 HL105759/HL/NHLBI NIH HHS/United States
- P01HL085577/HL/NHLBI NIH HHS/United States
- R01HL067245/HL/NHLBI NIH HHS/United States
- F31 HL123309/HL/NHLBI NIH HHS/United States
- R01HL101217/HL/NHLBI NIH HHS/United States
- R37 HL091102/HL/NHLBI NIH HHS/United States
- R37HL091102/HL/NHLBI NIH HHS/United States
- R01 HL113647/HL/NHLBI NIH HHS/United States
- R01 HL101217/HL/NHLBI NIH HHS/United States
- R01HL105759/HL/NHLBI NIH HHS/United States
- R01HL113647/HL/NHLBI NIH HHS/United States
- T32 GM007752/GM/NIGMS NIH HHS/United States
- R01 HL067245/HL/NHLBI NIH HHS/United States
- P01 HL085577/HL/NHLBI NIH HHS/United States
- R01 HL113656/HL/NHLBI NIH HHS/United States
- R01HL087023/HL/NHLBI NIH HHS/United States
- R01HL122525/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

