Free Energy Diagram for the Heterogeneous Enzymatic Hydrolysis of Glycosidic Bonds in Cellulose
- PMID: 26183776
- PMCID: PMC4571971
- DOI: 10.1074/jbc.M115.659656
Free Energy Diagram for the Heterogeneous Enzymatic Hydrolysis of Glycosidic Bonds in Cellulose
Abstract
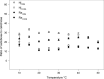
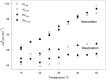
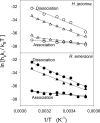
Kinetic and thermodynamic data have been analyzed according to transition state theory and a simplified reaction scheme for the enzymatic hydrolysis of insoluble cellulose. For the cellobiohydrolase Cel7A from Hypocrea jecorina (Trichoderma reesei), we were able to measure or collect relevant values for all stable and activated complexes defined by the reaction scheme and hence propose a free energy diagram for the full heterogeneous process. For other Cel7A enzymes, including variants with and without carbohydrate binding module (CBM), we obtained activation parameters for the association and dissociation of the enzyme-substrate complex. The results showed that the kinetics of enzyme-substrate association (i.e. formation of the Michaelis complex) was almost entirely entropy-controlled and that the activation entropy corresponded approximately to the loss of translational and rotational degrees of freedom of the dissolved enzyme. This implied that the transition state occurred early in the path where the enzyme has lost these degrees of freedom but not yet established extensive contact interactions in the binding tunnel. For dissociation, a similar analysis suggested that the transition state was late in the path where most enzyme-substrate contacts were broken. Activation enthalpies revealed that the rate of dissociation was far more temperature-sensitive than the rates of both association and the inner catalytic cycle. Comparisons of one- and two-domain variants showed that the CBM had no influence on the transition state for association but increased the free energy barrier for dissociation. Hence, the CBM appeared to promote the stability of the complex by delaying dissociation rather than accelerating association.
Keywords: Hypocrea jecorina; Rasamsonia emersonii; carbohydrate-binding protein; cellulase; enzyme kinetics; enzyme mechanism; thermodynamics.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
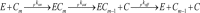


Similar articles
-
Temperature Effects on Kinetic Parameters and Substrate Affinity of Cel7A Cellobiohydrolases.J Biol Chem. 2015 Sep 4;290(36):22193-202. doi: 10.1074/jbc.M115.658930. Epub 2015 Jul 16. J Biol Chem. 2015. PMID: 26183777 Free PMC article.
-
Predominant Nonproductive Substrate Binding by Fungal Cellobiohydrolase I and Implications for Activity Improvement.Biotechnol J. 2019 Mar;14(3):e1700712. doi: 10.1002/biot.201700712. Epub 2018 Jun 19. Biotechnol J. 2019. PMID: 29781240
-
Kinetics of cellobiohydrolase (Cel7A) variants with lowered substrate affinity.J Biol Chem. 2014 Nov 21;289(47):32459-68. doi: 10.1074/jbc.M114.604264. Epub 2014 Sep 30. J Biol Chem. 2014. PMID: 25271162 Free PMC article.
-
[Mechanisms and regulation of enzymatic hydrolysis of cellulose in filamentous fungi: classical cases and new models].Rev Iberoam Micol. 2015 Jan-Mar;32(1):1-12. doi: 10.1016/j.riam.2013.10.009. Epub 2014 Mar 7. Rev Iberoam Micol. 2015. PMID: 24607657 Review. Spanish.
-
Enzyme free energy profiles: Can substrate binding be nonspontaneous? Can ground state interactions enhance catalysis?Biophys Chem. 2021 Jul;274:106606. doi: 10.1016/j.bpc.2021.106606. Epub 2021 Apr 28. Biophys Chem. 2021. PMID: 33945990 Review.
Cited by
-
Substrate binding in the processive cellulase Cel7A: Transition state of complexation and roles of conserved tryptophan residues.J Biol Chem. 2020 Feb 7;295(6):1454-1463. doi: 10.1074/jbc.RA119.011420. Epub 2019 Dec 17. J Biol Chem. 2020. PMID: 31848226 Free PMC article.
-
Computing Cellulase Kinetics with a Two-Domain Linear Interaction Energy Approach.ACS Omega. 2021 Jan 6;6(2):1547-1555. doi: 10.1021/acsomega.0c05361. eCollection 2021 Jan 19. ACS Omega. 2021. PMID: 33490814 Free PMC article.
-
Integrating electromagnetic cancer stress with immunotherapy: a therapeutic paradigm.Front Oncol. 2024 Aug 6;14:1417621. doi: 10.3389/fonc.2024.1417621. eCollection 2024. Front Oncol. 2024. PMID: 39165679 Free PMC article.
-
Engineering enhanced cellobiohydrolase activity.Nat Commun. 2018 Mar 22;9(1):1186. doi: 10.1038/s41467-018-03501-8. Nat Commun. 2018. PMID: 29567941 Free PMC article.
-
Enzymatic processing of lignocellulosic biomass: principles, recent advances and perspectives.J Ind Microbiol Biotechnol. 2020 Oct;47(9-10):623-657. doi: 10.1007/s10295-020-02301-8. Epub 2020 Aug 25. J Ind Microbiol Biotechnol. 2020. PMID: 32840713 Free PMC article. Review.
References
-
- Gelb M. H., Min J. H., Jain M. K. (2000) Do membrane-bound enzymes access their substrates from the membrane or aqueous phase: interfacial versus non-interfacial enzymes. Biochim. Biophys. Acta 1488, 20–27 - PubMed
-
- Illanes A., Wilson L., Vera C. (2013) Problem Solving in Enzyme Biocatalysis, John Wiley and Sons, Chichester, UK
-
- Kirk O., Borchert T. V., Fuglsang C. C. (2002) Industrial enzyme applications. Curr. Opin. Biotechnol. 13, 345–351 - PubMed
-
- Vocadlo D. J., Davies G. J. (2008) Mechanistic insights into glycosidase chemistry. Curr. Opin. Chem. Biol. 12, 539–555 - PubMed
-
- Withers S. G. (2001) Mechanisms of glycosyl transferases and hydrolases. Carbohydr. Polym. 44, 325–337
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

