Non-Nucleosidic Analogues of Polyaminonucleosides and Their Influence on Thermodynamic Properties of Derived Oligonucleotides
- PMID: 26184145
- PMCID: PMC6332422
- DOI: 10.3390/molecules200712652
Non-Nucleosidic Analogues of Polyaminonucleosides and Their Influence on Thermodynamic Properties of Derived Oligonucleotides
Abstract
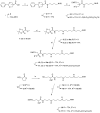
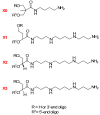
The rationale for the synthesis of cationic modified nucleosides is higher expected nuclease resistance and potentially better cellular uptake due to an overall reduced negative charge based on internal charge compensation. Due to the ideal distance between cationic groups, polyamines are perfect counterions for oligodeoxyribonucleotides. We have synthesized non-nucleosidic analogues built from units that carry different diol structures instead of sugar residues and functionalized with polyamines. The non-nucleosidic analogues were attached as internal or 5'-terminal modifications in oligodeoxyribonucleotide strands. The thermodynamic studies of these polyaminooligonucleotide analogues revealed stabilizing or destabilizing effects that depend on the linker or polyamine used.
Keywords: duplex DNA; non-nucleosidic analogue; oligonucleotide; polyaminonucleoside analogue; putrescine; spermine; thermodynamic stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
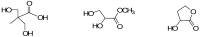

Similar articles
-
Selectivity of polyamines on the stability of RNA-DNA hybrids containing phosphodiester and phosphorothioate oligodeoxyribonucleotides.Biochemistry. 1999 Aug 17;38(33):10775-84. doi: 10.1021/bi990180t. Biochemistry. 1999. PMID: 10451373
-
Self-assembly of an oligodeoxyribonucleotide harboring the estrogen response element in the presence of polyamines: ionic, structural, and DNA sequence specificity effects.Biomacromolecules. 2000 Fall;1(3):339-49. doi: 10.1021/bm000010s. Biomacromolecules. 2000. PMID: 11710122
-
Versatile synthesis of oligodeoxyribonucleotide-oligospermine conjugates.Nat Protoc. 2007;2(6):1360-7. doi: 10.1038/nprot.2007.177. Nat Protoc. 2007. PMID: 17545974
-
Design and synthesis of triazolyl-donor/acceptor unnatural nucleosides and oligonucleotide probes containing triazolyl-phenanthrene nucleoside.Curr Protoc Nucleic Acid Chem. 2014 Sep 8;58:1.32.1-27. doi: 10.1002/0471142700.nc0132s58. Curr Protoc Nucleic Acid Chem. 2014. PMID: 25199635 Review.
-
Modulation of learning and memory by natural polyamines.Pharmacol Res. 2016 Oct;112:99-118. doi: 10.1016/j.phrs.2016.03.023. Epub 2016 Mar 22. Pharmacol Res. 2016. PMID: 27015893 Review.
References
-
- Prakash T.P., Barawkar D.A., Vaijayanti K., Ganesh K.N. Synthesis of site-specific oligonucleotide-polyamine conjugates. Bioorg. Med. Chem. Lett. 1994;4:1733–1738. doi: 10.1016/S0960-894X(00)80371-6. - DOI
-
- Horn T., Chaturvedi S., Balasubramaniam T.N., Letsinger R.L. Oligonucleotides with alternating anionic and cationic phosphoramidate linkages: Synthesis and hybridization of stereo-uniform isomers. Tetrahedron Lett. 1996;37:743–746. doi: 10.1016/0040-4039(95)02309-7. - DOI
-
- Letsinger R.L., Singman C.N., Histand G., Salunkhe M. Cationic oligonucleotides. J. Am. Chem. Soc. 1988;110:4470–4471. doi: 10.1021/ja00221a089. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

