Sirtuin 1 suppresses mitochondrial dysfunction of ischemic mouse livers in a mitofusin 2-dependent manner
- PMID: 26184910
- PMCID: PMC4716309
- DOI: 10.1038/cdd.2015.96
Sirtuin 1 suppresses mitochondrial dysfunction of ischemic mouse livers in a mitofusin 2-dependent manner
Abstract
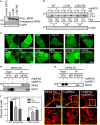
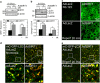
Ischemia/reperfusion (I/R) injury is a major cause of morbidity and mortality after liver surgery. The role of Sirtuin 1 (SIRT1) in hepatic I/R injury remains elusive. Using human and mouse livers, we investigated the effects of I/R on hepatocellular SIRT1. SIRT1 expression was significantly decreased after I/R. Genetic overexpression or pharmacological activation of SIRT1 markedly suppressed defective autophagy, onset of the mitochondrial permeability transition, and hepatocyte death after I/R, whereas SIRT1-null hepatocytes exhibited increased sensitivity to I/R injury. Biochemical approaches revealed that SIRT1 interacts with mitofusin-2 (MFN2). Furthermore, MFN2, but not MFN1, was deacetylated by SIRT1. Moreover, SIRT1 overexpression substantially increased autophagy in wild-type cells, but not in MFN2-deficient cells. Thus, our results demonstrate that the loss of SIRT1 causes a sequential chain of defective autophagy, mitochondrial dysfunction, and hepatocyte death after I/R.
Figures






References
-
- 1Kim J-S, He L, Qian T, Lemasters JJ. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr Mol Med 2003; 3: 527–535. - PubMed
-
- 2Kim J-S, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun 2003; 304: 463–470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

