Long-term results of carmustine wafer implantation for newly diagnosed glioblastomas: a controlled propensity-matched analysis of a French multicenter cohort
- PMID: 26185110
- PMCID: PMC4633930
- DOI: 10.1093/neuonc/nov126
Long-term results of carmustine wafer implantation for newly diagnosed glioblastomas: a controlled propensity-matched analysis of a French multicenter cohort
Abstract
Background: The standard of care for newly diagnosed glioblastoma is maximal safe surgical resection, followed by chemoradiation therapy. We assessed carmustine wafer implantation efficacy and safety when used in combination with standard care.
Methods: Included were adult patients with (n = 354, implantation group) and without (n = 433, standard group) carmustine wafer implantation during first surgical resection followed by chemoradiation standard protocol. Multivariate and case-matched analyses (controlled propensity-matched cohort, 262 pairs of patients) were conducted.
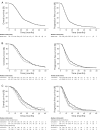
Results: The median progression-free survival was 12.0 months (95% CI: 10.7-12.6) in the implantation group and 10.0 months (9.0-10.0) in the standard group and the median overall survival was 20.4 months (19.0-22.7) and 18.0 months (17.0-19.0), respectively. Carmustine wafer implantation was independently associated with longer progression-free survival in patients with subtotal/total surgical resection in the whole series (adjusted hazard ratio [HR], 0.76 [95% CI: 0.63-0.92], P = .005) and after propensity matching (HR, 0.74 [95% CI: 0.60-0.92], P = .008), whereas no significant difference was found for overall survival (HR, 0.95 [0.80-1.13], P = .574; HR, 1.06 [0.87-1.29], P = .561, respectively). Surgical resection at progression whether alone or combined with carmustine wafer implantation was independently associated with longer overall survival in the whole series (HR, 0.58 [0.44-0.76], P < .0001; HR, 0.54 [0.41-0.70], P < .0001, respectively) and after propensity matching (HR, 0.56 [95% CI: 0.40-0.78], P < .0001; HR, 0.46 [95% CI: 0.33-0.64], P < .0001, respectively). The higher postoperative infection rate in the implantation group did not affect survival.
Conclusions: Carmustine wafer implantation during surgical resection followed by the standard chemoradiation protocol for newly diagnosed glioblastoma in adults resulted in a significant progression-free survival benefit.
Keywords: carmustine wafers; chemoradiotherapy; glioblastoma; surgery; survival.
© The Author(s) 2015. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Ricard D, Idbaih A, Ducray F, et al. Primary brain tumours in adults. Lancet. 2012;379(9830):1984–1996. - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

