Kidney Disease Caused by Dysregulation of the Complement Alternative Pathway: An Etiologic Approach
- PMID: 26185203
- PMCID: PMC4657846
- DOI: 10.1681/ASN.2015020184
Kidney Disease Caused by Dysregulation of the Complement Alternative Pathway: An Etiologic Approach
Abstract
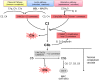
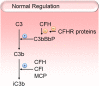
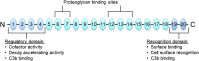
Kidney diseases caused by genetic or acquired dysregulation of the complement alternative pathway (AP) are traditionally classified on the basis of clinical presentation (atypical hemolytic uremic syndrome as thrombotic microangiopathy), biopsy appearance (dense deposit disease and C3 GN), or clinical course (atypical postinfectious GN). Each is characterized by an inappropriate activation of the AP, eventuating in renal damage. The clinical diversity of these disorders highlights important differences in the triggers, the sites and intensity of involvement, and the outcome of the AP dysregulation. Nevertheless, we contend that these diseases should be grouped as disorders of the AP and classified on an etiologic basis. In this review, we define different pathophysiologic categories of AP dysfunction. The precise identification of the underlying abnormality is the key to predict the response to immune suppression, plasma infusion, and complement-inhibitory drugs and the outcome after transplantation. In a patient with presumed dysregulation of the AP, the collaboration of the clinician, the renal pathologist, and the biochemical and genetic laboratory is very much encouraged, because this enables the elucidation of both the underlying pathogenesis and the optimal therapeutic approach.
Keywords: GN; complement; hemolytic uremic syndrome.
Copyright © 2015 by the American Society of Nephrology.
Figures



References
-
- Thurman JM, Holers VM: The central role of the alternative complement pathway in human disease. J Immunol 176: 1305–1310, 2006 - PubMed
-
- Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M, Doyle M, Fakhouri F, Fervenza FC, Fogo AB, Frémeaux-Bacchi V, Gale DP, Goicoechea de Jorge E, Griffin G, Harris CL, Holers VM, Johnson S, Lavin PJ, Medjeral-Thomas N, Paul Morgan B, Nast CC, Noel LH, Peters DK, Rodríguez de Córdoba S, Servais A, Sethi S, Song WC, Tamburini P, Thurman JM, Zavros M, Cook HT: C3 glomerulopathy: Consensus report. Kidney Int 84: 1079–1089, 2013 - PMC - PubMed
-
- Sethi S, Fervenza FC: Pathology of renal diseases associated with dysfunction of the alternative pathway of complement: C3 glomerulopathy and atypical hemolytic uremic syndrome (aHUS). Semin Thromb Hemost 40: 416–421, 2014 - PubMed
-
- Bomback AS, Appel GB: Pathogenesis of the C3 glomerulopathies and reclassification of MPGN. Nat Rev Nephrol 8: 634–642, 2012 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

