Landscape of Familial Isolated and Young-Onset Pituitary Adenomas: Prospective Diagnosis in AIP Mutation Carriers
- PMID: 26186299
- PMCID: PMC4570169
- DOI: 10.1210/jc.2015-1869
Landscape of Familial Isolated and Young-Onset Pituitary Adenomas: Prospective Diagnosis in AIP Mutation Carriers
Abstract
Context: Familial isolated pituitary adenoma (FIPA) due to aryl hydrocarbon receptor interacting protein (AIP) gene mutations is an autosomal dominant disease with incomplete penetrance. Clinical screening of apparently unaffected AIP mutation (AIPmut) carriers could identify previously unrecognized disease.
Objective: To determine the AIP mutational status of FIPA and young pituitary adenoma patients, analyzing their clinical characteristics, and to perform clinical screening of apparently unaffected AIPmut carrier family members.
Design: This was an observational, longitudinal study conducted over 7 years.
Setting: International collaborative study conducted at referral centers for pituitary diseases.
Participants: FIPA families (n 216) and sporadic young-onset (30 y) pituitary adenoma patients (n 404) participated in the study.
Interventions: We performed genetic screening of patients for AIPmuts, clinical assessment of their family members, and genetic screening for somatic GNAS1 mutations and the germline FGFR4 p.G388R variant.
Main outcome measure(s): We assessed clinical disease in mutation carriers, comparison of characteristics of AIPmut positive and negative patients, results of GNAS1, and FGFR4 analysis.
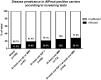
Results: Thirty-seven FIPA families and 34 sporadic patients had AIPmuts. Patients with truncating AIPmuts had a younger age at disease onset and diagnosis, compared with patients with nontruncating AIPmuts. Somatic GNAS1 mutations were absent in tumors from AIPmut-positive patients, and the studied FGFR4 variant did not modify the disease behavior or penetrance in AIPmut-positive individuals. A total of 164 AIPmut-positive unaffected family members were identified; pituitary disease was detected in 18 of those who underwent clinical screening.
Conclusions: A quarter of the AIPmut carriers screened were diagnosed with pituitary disease, justifying this screening and suggesting a variable clinical course for AIPmut-positive pituitary adenomas.
Trial registration: ClinicalTrials.gov NCT00461188.
Figures

References
-
- Vierimaa O, Georgitsi M, Lehtonen R, et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312:1228–1230. - PubMed
-
- Daly AF, Vanbellinghen JF, Khoo SK, et al. Aryl hydrocarbon receptor-interacting protein gene mutations in familial isolated pituitary adenomas: analysis in 73 families. J Clin Endocrinol Metab. 2007;92:1891–1896. - PubMed
-
- Leontiou CA, Gueorguiev M, van der Spuy J, et al. The role of the aryl hydrocarbon receptor-interacting protein gene in familial and sporadic pituitary adenomas. J Clin Endocrinol Metab. 2008;93:2390–2401. - PubMed
-
- Daly AF, Tichomirowa MA, Petrossians P, et al. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J Clin Endocrinol Metab. 2010;95:E373–E383. - PubMed
-
- Cazabat L, Libe R, Perlemoine K, et al. Germline inactivating mutations of the aryl hydrocarbon receptor-interacting protein gene in a large cohort of sporadic acromegaly: mutations are found in a subset of young patients with macroadenomas. Eur J Endocrinol. 2007;157:1–8. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

