Evidence for the involvement of gamma delta T cells in the immune response in Rasmussen encephalitis
- PMID: 26186920
- PMCID: PMC4506578
- DOI: 10.1186/s12974-015-0352-2
Evidence for the involvement of gamma delta T cells in the immune response in Rasmussen encephalitis
Abstract
Background: Rasmussen encephalitis (RE) is a rare neuroinflammatory disease characterized by intractable seizures and progressive atrophy on one side of the cerebrum. Perivascular cuffing and clusters of T cells in the affected cortical hemisphere are indicative of an active cellular immune response.
Methods: Peripheral blood mononuclear cells (PBMCs) and brain-infiltrating lymphocytes (BILs) were isolated from 20 RE surgery specimens by standard methods, and CD3(+) T cell populations were analyzed by flow cytometry. Gamma delta T cell receptor spectratyping was carried out by nested PCR of reversed transcribed RNA extracted from RE brain tissue, followed by high resolution capillary electrophoresis. A MiSeq DNA sequencing platform was used to sequence the third complementarity determining region (CDR3) of δ1 chains.
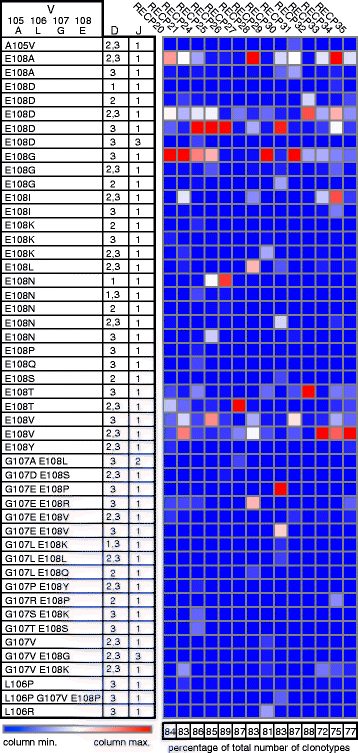
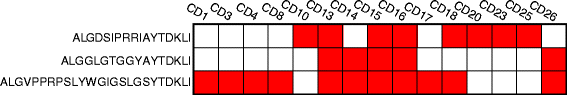
Results: CD3(+) BILs from all of the RE brain specimens comprised both αβ and γδ T cells. The median αβ:γδ ratio was 1.9 (range 0.58-5.2) compared with a median ratio of 7.7 (range 2.7-40.8) in peripheral blood from the same patients. The αβ T cells isolated from brain tissue were predominantly CD8(+), and the majority of γδ T cells were CD4(-) CD8(-). Staining for the early activation marker CD69 showed that a fraction of the αβ and γδ T cells in the BILs were activated (median 42%; range 13-91%, and median 47%; range 14-99%, respectively). Spectratyping T cell receptor (TCR) Vδ1-3 chains from 14 of the RE brain tissue specimens indicated that the γδ T cell repertoire was relatively restricted. Sequencing δ1 chain PCR fragments revealed that the same prevalent CDR3 sequences were found in all of the brain specimens. These CDR3 sequences were also detected in brain tissue from 15 focal cortical dysplasia (FCD) cases.
Conclusion: Neuroinflammation in RE involves both activated αβ and γδ T cells. The presence of γδ T cells with identical TCR δ1 chain CDR3 sequences in all of the brain specimens examined suggests that a non-major histocompatibility complex (MHC)-restricted immune response to the same antigen(s) is involved in the etiology of RE. The presence of the same δ1 clones in CD brain implies the involvement of a common inflammatory pathway in both diseases.
Figures





References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

