Leukemic marrow infiltration reveals a novel role for Egr3 as a potent inhibitor of normal hematopoietic stem cell proliferation
- PMID: 26186938
- PMCID: PMC4574014
- DOI: 10.1182/blood-2015-01-623645
Leukemic marrow infiltration reveals a novel role for Egr3 as a potent inhibitor of normal hematopoietic stem cell proliferation
Abstract
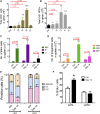
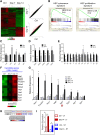
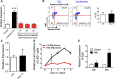
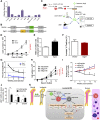
Cytopenias resulting from the impaired generation of normal blood cells from hematopoietic precursors are important contributors to morbidity and mortality in patients with leukemia. However, the process by which normal hematopoietic cells are overtaken by emerging leukemia cells and how different subsets of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) are distinctly influenced during leukemic cell infiltration is poorly understood. To investigate these important questions, we used a robust nonirradiated mouse model of human MLL-AF9 leukemia to examine the suppression of HSCs and HPCs during leukemia cell expansion in vivo. Among all the hematopoietic subsets, long-term repopulating HSCs were the least reduced, whereas megakaryocytic-erythroid progenitors were the most significantly suppressed. Notably, nearly all of the HSCs were forced into a noncycling state in leukemic marrow at late stages, but their reconstitution potential appeared to be intact upon transplantation into nonleukemic hosts. Gene expression profiling and further functional validation revealed that Egr3 was a strong limiting factor for the proliferative potential of HSCs. Therefore, this study provides not only a molecular basis for the more tightened quiescence of HSCs in leukemia, but also a novel approach for defining functional regulators of HSCs in disease.
© 2015 by The American Society of Hematology.
Figures



Comment in
-
"Blood feuds".Blood. 2015 Sep 10;126(11):1264-5. doi: 10.1182/blood-2015-07-659540. Blood. 2015. PMID: 26359429 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

