Lost after translation: insights from pulmonary surfactant for understanding the role of alveolar epithelial dysfunction and cellular quality control in fibrotic lung disease
- PMID: 26186947
- PMCID: PMC4572416
- DOI: 10.1152/ajplung.00139.2015
Lost after translation: insights from pulmonary surfactant for understanding the role of alveolar epithelial dysfunction and cellular quality control in fibrotic lung disease
Abstract
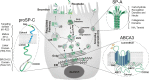
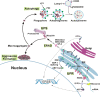
Dating back nearly 35 years ago to the Witschi hypothesis, epithelial cell dysfunction and abnormal wound healing have reemerged as central concepts in the pathophysiology of idiopathic pulmonary fibrosis (IPF) in adults and in interstitial lung disease in children. Alveolar type 2 (AT2) cells represent a metabolically active compartment in the distal air spaces responsible for pulmonary surfactant biosynthesis and function as a progenitor population required for maintenance of alveolar integrity. Rare mutations in surfactant system components have provided new clues to understanding broader questions regarding the role of AT2 cell dysfunction in the pathophysiology of fibrotic lung diseases. Drawing on data generated from a variety of model systems expressing disease-related surfactant component mutations [surfactant proteins A and C (SP-A and SP-C); the lipid transporter ABCA3], this review will examine the concept of epithelial dysfunction in fibrotic lung disease, provide an update on AT2 cell and surfactant biology, summarize cellular responses to mutant surfactant components [including endoplasmic reticulum (ER) stress, mitochondrial dysfunction, and intrinsic apoptosis], and examine quality control pathways (unfolded protein response, the ubiquitin-proteasome system, macroautophagy) that can be utilized to restore AT2 homeostasis. This integrated response and its derangement will be placed in the context of cell stress and quality control signatures found in patients with familial or sporadic IPF as well as non-surfactant-related AT2 cell dysfunction syndromes associated with a fibrotic lung phenotype. Finally, the need for targeted therapeutic strategies for pulmonary fibrosis that address epithelial ER stress, its downstream signaling, and cell quality control are discussed.
Keywords: alveolar type 2 cells; cell quality control; epithelial ER stress; fibrotic lung disease; surfactant proteins.
Copyright © 2015 the American Physiological Society.
Figures

References
-
- Abou TR, Jaubert F, Emond S, Le Bourgeois M, Epaud R, Karila C, Feldmann D, Scheinmann P, de Blic J. Familial interstitial disease with I73T mutation: a mid- and long-term study. Pediatr Pulmonol 44: 167–175, 2009. - PubMed
-
- Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, Yanagisawa H, Kobayashi K, Tsurushige C, Kawaishi M, Kamiya N, Hirano J, Odaka M, Morikawa T, Nishimura SL, Kawabata Y, Hano H, Nakayama K, Kuwano K. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 304: L56–L69, 2013. - PubMed
-
- Armanios MY, Chen JJL, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 356: 1317–1326, 2007. - PubMed
-
- Atochina-Vasserman EN, Bates SR, Zhang P, Abramova H, Zhang Z, Gonzales L, Tao JQ, Gochuico BR, Gahl W, Guo CJ, Gow AJ, Beers MF, Guttentag S. Early alveolar epithelial dysfunction promotes lung inflammation in a mouse model of Hermansky-Pudlak syndrome. Am J Respir Crit Care Med 184: 449–458, 2011. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

