A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity
- PMID: 26187233
- PMCID: PMC4841173
- DOI: 10.1093/gerona/glv057
A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity
Erratum in
-
Errata.J Gerontol A Biol Sci Med Sci. 2016 Jun;71(6):839-40. doi: 10.1093/gerona/glw056. Epub 2016 Apr 8. J Gerontol A Biol Sci Med Sci. 2016. PMID: 27059599 Free PMC article. No abstract available.
Abstract
Background: Caloric restriction (CR), energy intake reduced below ad libitum (AL) intake, increases life span in many species. The implications for humans can be clarified by randomized controlled trials of CR.
Methods: To determine CR's feasibility, safety, and effects on predictors of longevity, disease risk factors, and quality of life in nonobese humans aged 21-51 years, 218 persons were randomized to a 2-year intervention designed to achieve 25% CR or to AL diet. Outcomes were change from baseline resting metabolic rate adjusted for weight change ("RMR residual") and core temperature (primary); plasma triiodothyronine (T3) and tumor necrosis factor-α (secondary); and exploratory physiological and psychological measures.
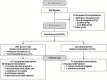
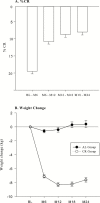
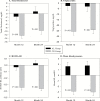
Results: Body mass index averaged 25.1 (range: 21.9-28.0 kg/m(2)). Eighty-two percent of CR and 95% of AL participants completed the protocol. The CR group achieved 11.7±0.7 %CR (mean ± standard error) and maintained 10.4±0.4% weight loss. Weight change in AL was negligible. RMR residual decreased significantly more in CR than AL at 12 months (p = .04) but not 24 months (M24). Core temperature change differed little between groups. T3 decreased more in CR at M12 and M24 (p < .001), while tumor necrosis factor-α decreased significantly more only at M24 (p = .02). CR had larger decreases in cardiometabolic risk factors and in daily energy expenditure adjusted for weight change, without adverse effects on quality of life.
Conclusions: Sustained CR is feasible in nonobese humans. The effects of the achieved CR on correlates of human survival and disease risk factors suggest potential benefits for aging-related outcomes that could be elucidated by further human studies.
Trial registration: ClinicalTrials.gov NCT00427193.
Keywords: Biomarkers; Caloric restriction; Metabolism; Nutrition; Risk factors.
Published by Oxford University Press on behalf of the Gerontological Society of America 2015.
Figures

Comment in
-
Two-Year Trial of Human Caloric Restriction.J Gerontol A Biol Sci Med Sci. 2015 Sep;70(9):1095-6. doi: 10.1093/gerona/glv100. Epub 2015 Jul 17. J Gerontol A Biol Sci Med Sci. 2015. PMID: 26187232 No abstract available.
References
-
- Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32:159–221. doi:10.1016/j.mam.2011.07.001 - PubMed
-
- Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J Gerontol A Biol Sci Med Sci. 2003;58:212–219. - PubMed

