Macrolides rapidly inhibit red blood cell invasion by the human malaria parasite, Plasmodium falciparum
- PMID: 26187647
- PMCID: PMC4506589
- DOI: 10.1186/s12915-015-0162-0
Macrolides rapidly inhibit red blood cell invasion by the human malaria parasite, Plasmodium falciparum
Abstract
Background: Malaria invasion of red blood cells involves multiple parasite-specific targets that are easily accessible to inhibitory compounds, making it an attractive target for antimalarial development. However, no current antimalarial agents act against host cell invasion.
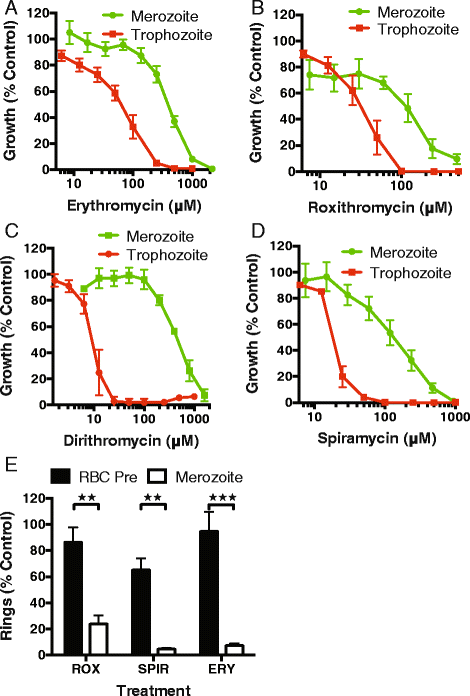
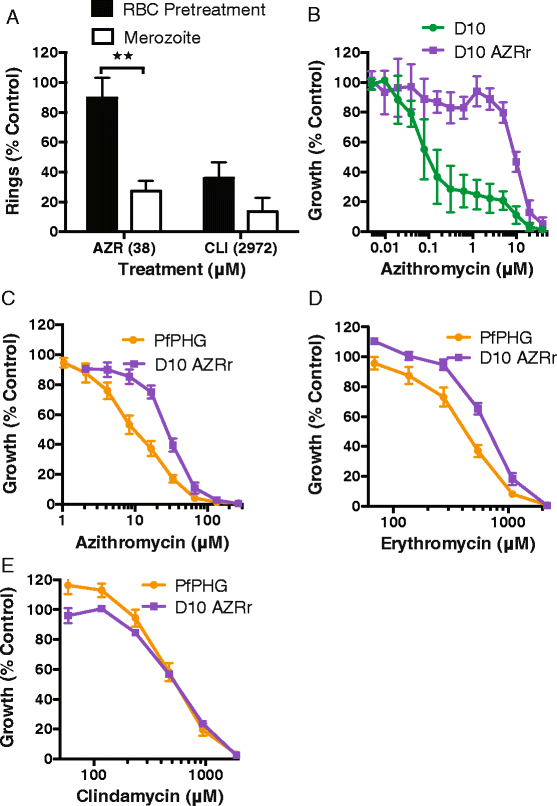
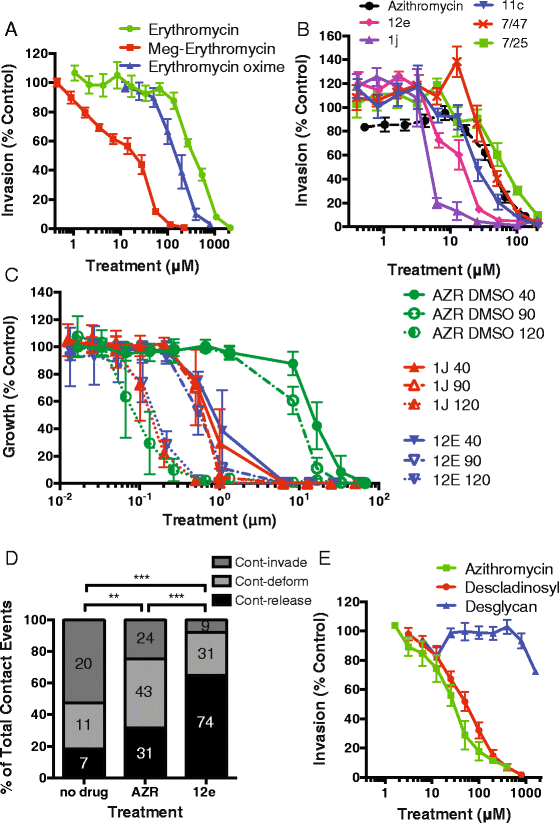
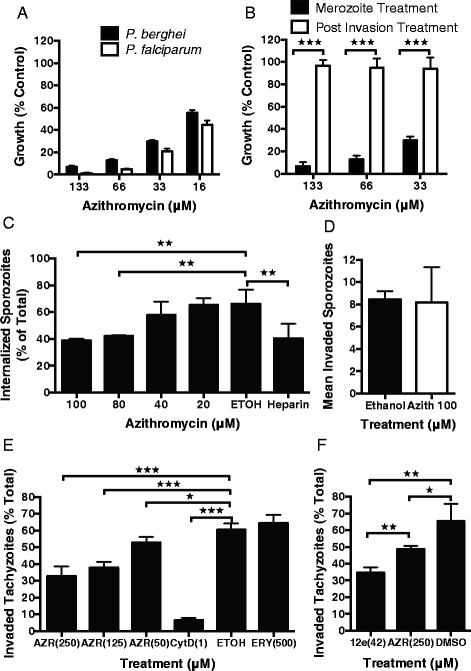
Results: Here, we demonstrate that the clinically used macrolide antibiotic azithromycin, which is known to kill human malaria asexual blood-stage parasites by blocking protein synthesis in their apicoplast, is also a rapid inhibitor of red blood cell invasion in human (Plasmodium falciparum) and rodent (P. berghei) malarias. Multiple lines of evidence demonstrate that the action of azithromycin in inhibiting parasite invasion of red blood cells is independent of its inhibition of protein synthesis in the parasite apicoplast, opening up a new strategy to develop a single drug with multiple parasite targets. We identified derivatives of azithromycin and erythromycin that are better invasion inhibitors than parent compounds, offering promise for development of this novel antimalarial strategy.
Conclusions: Safe and effective macrolide antibiotics with dual modalities could be developed to combat malaria and reduce the parasite's options for resistance.
Figures
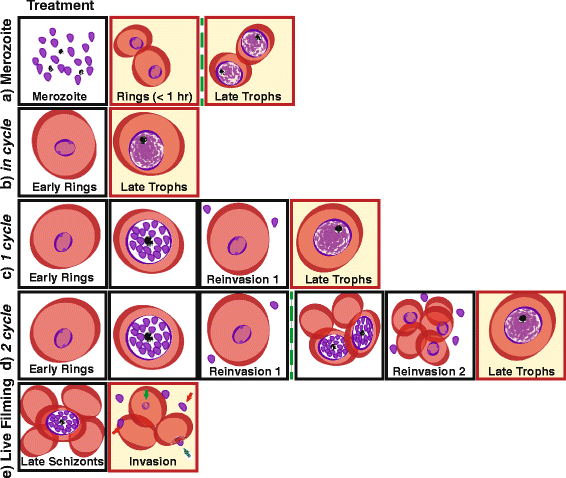
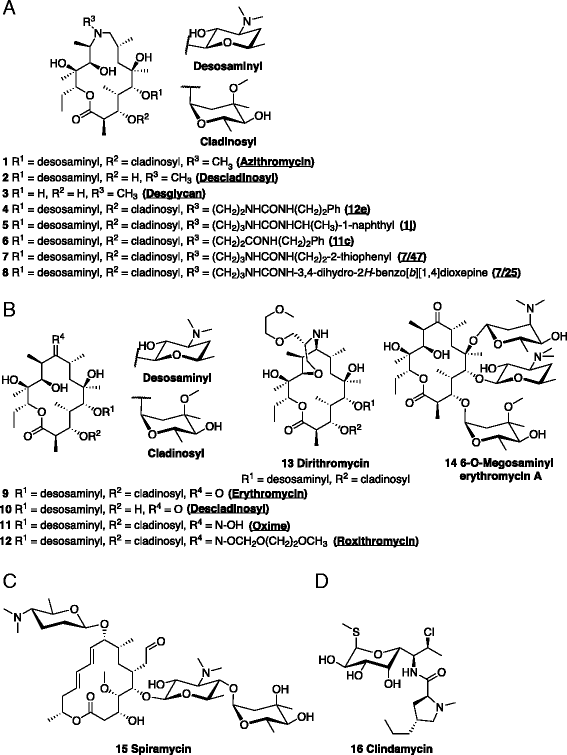
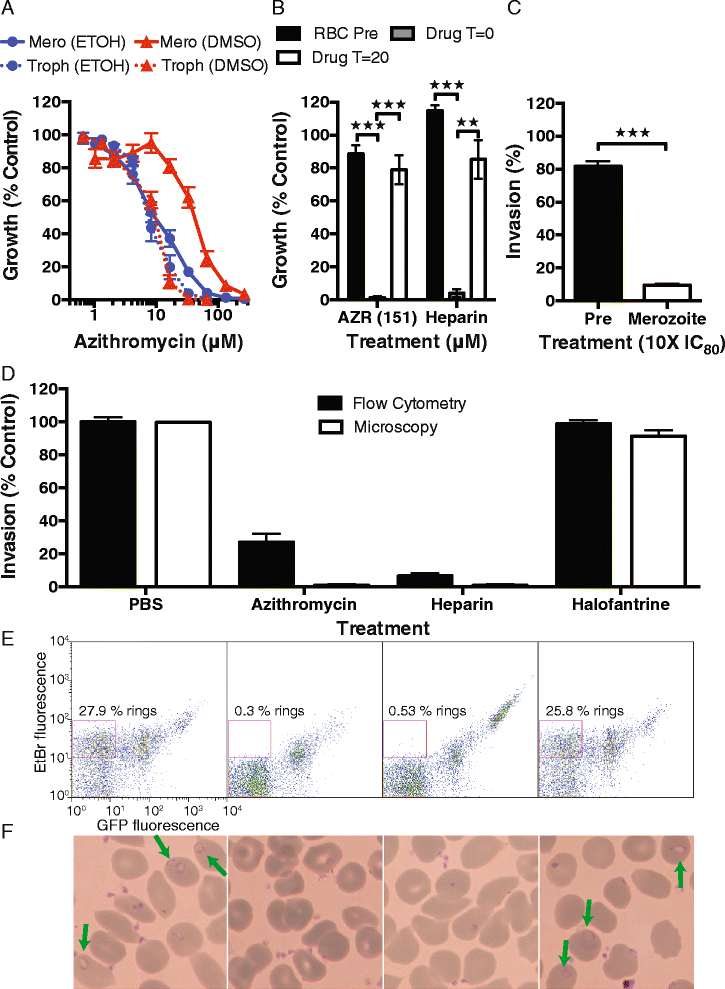
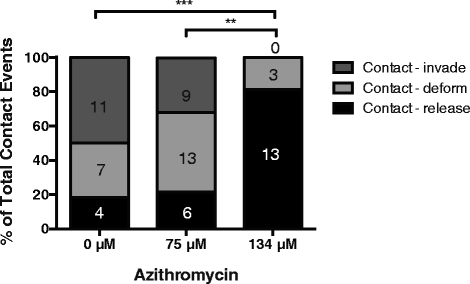
Comment in
-
Teaching old drugs new tricks to stop malaria invasion in its tracks.BMC Biol. 2015 Sep 8;13:72. doi: 10.1186/s12915-015-0185-6. BMC Biol. 2015. PMID: 26349580 Free PMC article.
References
-
- World Health Organization (WHO) World malaria report 2014. Geneva: WHO; 2014.
-
- Ferone R, Burchall JJ, Hitchings GH. Plasmodium berghei dihydrofolate reductase. Isolation, properties, and inhibition by antifolates. Mol Pharmacol. 1969;5:49–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

