Contribution of the platelet activating factor signaling pathway to cerebral microcirculatory dysfunction during experimental sepsis by ExoU producing Pseudomonas aeruginosa
- PMID: 26187894
- PMCID: PMC4626577
- DOI: 10.1093/femspd/ftv046
Contribution of the platelet activating factor signaling pathway to cerebral microcirculatory dysfunction during experimental sepsis by ExoU producing Pseudomonas aeruginosa
Abstract
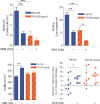
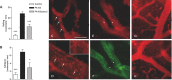
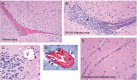
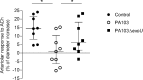
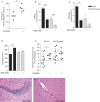
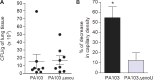
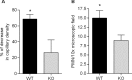
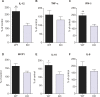
Intravital microscopy was used to assess the involvement of ExoU, a Pseudomonas aeruginosa cytotoxin with phospholipase A2 activity, in dysfunction of cerebral microcirculation during experimental pneumosepsis. Cortical vessels from mice intratracheally infected with low density of the ExoU-producing PA103 P. aeruginosa strain exhibited increased leukocyte rolling and adhesion to venule endothelium, decreased capillar density and impaired arteriolar response to vasoactive acetylcholine. These phenomena were mediated by the platelet activating factor receptor (PAFR) pathway because they were reversed in mice treated with a PAFR antagonist prior to infection. Brains from PA103-infected animals exhibited a perivascular inflammatory infiltration that was not detected in animals infected with an exoU deficient mutant or in mice treated with the PAFR antagonist and infected with the wild type bacteria. No effect on brain capillary density was detected in mice infected with the PAO1 P. aeruginosa strain, which do not produce ExoU. Finally, after PA103 infection, mice with a targeted deletion of the PAFR gene exhibited higher brain capillary density and lower leukocyte adhesion to venule endothelium, as well as lower increase of systemic inflammatory cytokines, when compared to wild-type mice. Altogether, our results establish a role for PAFR in mediating ExoU-induced cerebral microvascular failure in a murine model of sepsis.
Keywords: PAF–PAFR signaling pathway; Pseudomonas aeruginosa type III secretion toxin; cerebral intravital microscopy; cerebral microvascular failure; experimental Pseudomonas aeruginosa sepsis.
© FEMS 2015. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Araújo CV, Estato V, Tibiriçá E, et al. PPAR gamma activation protects the brain against microvascular dysfunction in sepsis. Microvasc Res. 2012;84:218–21. - PubMed
-
- Castor MG, Rezende BM, Resende CB, et al. Platelet-activating factor receptor plays a role in the pathogenesis of graft-versus-host disease by regulating leukocyte recruitment, tissue injury and lethality. J Leukocyte Biol. 2012;91:629–39. - PubMed
-
- Correa-Costa M, Andrade-Oliveira V, Braga TT, et al. Activation of platelet-activating factor receptor exacerbates renal inflammation and promotes fibrosis. Lab Invest. 2014;94:455–66. - PubMed
-
- De Backer D, Creteur J, Preiser JC, et al. Microvascular blood flow is altered in patients with sepsis. Am J Resp Crit Care. 2002;166:98–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

