Chimeric Antigen Receptor- and TCR-Modified T Cells Enter Main Street and Wall Street
- PMID: 26188068
- PMCID: PMC4507286
- DOI: 10.4049/jimmunol.1500751
Chimeric Antigen Receptor- and TCR-Modified T Cells Enter Main Street and Wall Street
Abstract
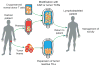
The field of adoptive cell transfer (ACT) is currently comprised of chimeric Ag receptor (CAR)- and TCR-engineered T cells and has emerged from principles of basic immunology to paradigm-shifting clinical immunotherapy. ACT of T cells engineered to express artificial receptors that target cells of choice is an exciting new approach for cancer, and it holds equal promise for chronic infection and autoimmunity. Using principles of synthetic biology, advances in immunology, and genetic engineering have made it possible to generate human T cells that display desired specificities and enhanced functionalities. Clinical trials in patients with advanced B cell leukemias and lymphomas treated with CD19-specific CAR T cells have induced durable remissions in adults and children. The prospects for the widespread availability of engineered T cells have changed dramatically given the recent entry of the pharmaceutical industry to this arena. In this overview, we discuss some of the challenges and opportunities that face the field of ACT.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures

References
-
- June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: A race to the finish line. Sci Transl Med. 2015;7:280ps287. - PubMed
-
- Riddell SR, Elliott M, Lewinsohn DA, Gilbert MJ, Wilson L, Manley SA, Lupton SD, Overell RW, Reynolds TC, Corey L, Greenberg PD. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2:216–223. - PubMed
-
- Scholler J, Brady T, Binder-Scholl G, Hwang WT, Plesa G, Hege K, Vogel A, Kalos M, Riley J, Deeks S, Mitsuyasu R, Bernstein W, Aronson N, Levine B, Bushman F, June C. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Science Translational Medicine. 2012;4:132Ra153. - PMC - PubMed
-
- Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O. Long-Term Control of HIV by CCR5 Delta32/Delta32 Stem-Cell Transplantation. New England Journal of Medicine. 2009;360:692–696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

