A plant alkaloid, veratridine, potentiates cancer chemosensitivity by UBXN2A-dependent inhibition of an oncoprotein, mortalin-2
- PMID: 26188124
- PMCID: PMC4695137
- DOI: 10.18632/oncotarget.4452
A plant alkaloid, veratridine, potentiates cancer chemosensitivity by UBXN2A-dependent inhibition of an oncoprotein, mortalin-2
Abstract
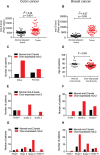
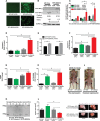
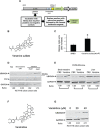
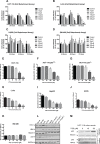
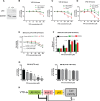
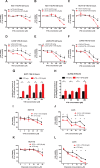
Veratridine (VTD), an alkaloid derived from the Liliaceae plant shows anti-tumor effects; however, its molecular targets have not been thoroughly studied. Using a high-throughput drug screen, we found that VTD enhances transactivation of UBXN2A, resulting in upregulation of UBXN2A in the cytoplasm, where UBXN2A binds and inhibits the oncoprotein mortalin-2 (mot-2). VTD-treated cancer cells undergo cell death in UBXN2A- and mot-2-dependent manners. The cytotoxic function of VTD is grade-dependent, and the combined treatment with a sub-optimal dose of the standard chemotherapy, 5-Fluorouracil (5-FU) and etoposide, demonstrated a synergistic effect, resulting in higher therapeutic efficacy. VTD influences the CD44+ stem cells, possibly through UBXN2A-dependent inhibition of mot-2. The VTD-dependent expression of UBXN2A is a potential candidate for designing novel strategies for colon cancer treatment because: 1) In 50% of colon cancer patients, UBXN2A protein levels in tumor tissues are significantly lower than those in the adjacent normal tissues. 2) Cytoplasmic expression of the mot-2 protein is very low in non-cancerous cells; thus, VTD can produce tumor-specific toxicity while normal cells remain intact. 3) Finally, VTD or its modified analogs offer a valuable adjuvant chemotherapy strategy to improve the efficacy of 5-FU-based chemotherapy for colon cancer patients harboring WT-p53.
Keywords: UBXN2A; chemotherapy; mortalin-2; p53; veratridine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kaul SC, Aida S, Yaguchi T, Kaur K, Wadhwa R. Activation of wild type p53 function by its mortalin-binding, cytoplasmically localizing carboxyl terminus peptides. J Biol Chem. 2005;280:39373–39379. - PubMed
-
- Wadhwa R, Takano S, Robert M, Yoshida A, Nomura H, Reddel RR, Mitsui Y, Kaul SC. Inactivation of tumor suppressor p53 by mot-2, a hsp70 family member. J Biol Chem. 1998;273:29586–29591. - PubMed
-
- Sane S, Abdullah A, Boudreau DA, Autenried RK, Gupta BK, Wang X, Wang H, Schlenker EH, Zhang D, Telleria C, Huang L, Chauhan SC, Rezvani K. Ubiquitin-like (UBX)-domain-containing protein, UBXN2A, promotes cell death by interfering with the p53-Mortalin interactions in colon cancer cells. Cell Death Dis. 2014;5:e1118. - PMC - PubMed
-
- Burbulla LF, Schelling C, Kato H, Rapaport D, Woitalla D, Schiesling C, Schulte C, Sharma M, Illig T, Bauer P, Jung S, Nordheim A, Schols L, Riess O, Kruger R. Dissecting the role of the mitochondrial chaperone mortalin in Parkinson's disease: functional impact of disease-related variants on mitochondrial homeostasis. Hum Mol Genet. 2010;19:4437–4452. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

