Regulation of integrin-mediated adhesions
- PMID: 26189062
- PMCID: PMC4639423
- DOI: 10.1016/j.ceb.2015.06.009
Regulation of integrin-mediated adhesions
Abstract
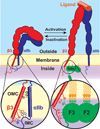
Integrins are heterodimeric transmembrane adhesion receptors that couple the actin cytoskeleton to the extracellular environment and bidirectionally relay signals across the cell membrane. These processes are critical for cell attachment, migration, differentiation, and survival, and therefore play essential roles in metazoan development, physiology, and pathology. Integrin-mediated adhesions are regulated by diverse factors, including the conformation-specific affinities of integrin receptors for their extracellular ligands, the clustering of integrins and their intracellular binding partners into discrete adhesive structures, mechanical forces exerted on the adhesion, and the intracellular trafficking of integrins themselves. Recent advances shed light onto how the interaction of specific intracellular proteins with the short cytoplasmic tails of integrins controls each of these activities.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

References
-
-
Zhu J, Zhu J, Springer TA. Complete integrin headpiece opening in eight steps. J Cell Biol. 2013;201:1053–1068. By soaking RGD ligands into closed αIIbβ3 headpiece crystals under various conditions the authors reveal 8 distinct conformational steps along the integrin activation and ligand binding pathway.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

