Radial and tangential migration of telencephalic somatostatin neurons originated from the mouse diagonal area
- PMID: 26189100
- PMCID: PMC4920861
- DOI: 10.1007/s00429-015-1086-8
Radial and tangential migration of telencephalic somatostatin neurons originated from the mouse diagonal area
Abstract
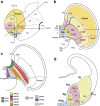
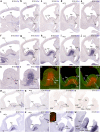
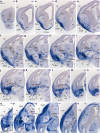
The telencephalic subpallium is the source of various GABAergic interneuron cohorts that invade the pallium via tangential migration. Based on genoarchitectonic studies, the subpallium has been subdivided into four major domains: striatum, pallidum, diagonal area and preoptic area (Puelles et al. 2013; Allen Developing Mouse Brain Atlas), and a larger set of molecularly distinct progenitor areas (Flames et al. 2007). Fate mapping, genetic lineage-tracing studies, and other approaches have suggested that each subpallial subdivision produces specific sorts of inhibitory interneurons, distinguished by differential peptidic content, which are distributed tangentially to pallial and subpallial target territories (e.g., olfactory bulb, isocortex, hippocampus, pallial and subpallial amygdala, striatum, pallidum, septum). In this report, we map descriptively the early differentiation and apparent migratory dispersion of mouse subpallial somatostatin-expressing (Sst) cells from E10.5 onward, comparing their topography with the expression patterns of the genes Dlx5, Gbx2, Lhx7-8, Nkx2.1, Nkx5.1 (Hmx3), and Shh, which variously label parts of the subpallium. Whereas some experimental results suggest that Sst cells are pallidal, our data reveal that many, if not most, telencephalic Sst cells derive from de diagonal area (Dg). Sst-positive cells initially only present at the embryonic Dg selectively populate radially the medial part of the bed nucleus striae terminalis (from paraseptal to amygdaloid regions) and part of the central amygdala; they also invade tangentially the striatum, while eschewing the globus pallidum and the preoptic area, and integrate within most cortical and nuclear pallial areas between E10.5 and E16.5.
Keywords: Cortex; Entopeduncular area; Forebrain interneurons; Medial ganglionic eminence; Pallidum; Preoptic area; Secondary prosencephalon; Striatum; Subpallium.
Figures









References
-
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

