A Genome-wide CRISPR Screen in Primary Immune Cells to Dissect Regulatory Networks
- PMID: 26189680
- PMCID: PMC4522370
- DOI: 10.1016/j.cell.2015.06.059
A Genome-wide CRISPR Screen in Primary Immune Cells to Dissect Regulatory Networks
Abstract
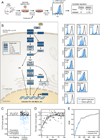
Finding the components of cellular circuits and determining their functions systematically remains a major challenge in mammalian cells. Here, we introduced genome-wide pooled CRISPR-Cas9 libraries into dendritic cells (DCs) to identify genes that control the induction of tumor necrosis factor (Tnf) by bacterial lipopolysaccharide (LPS), a key process in the host response to pathogens, mediated by the Tlr4 pathway. We found many of the known regulators of Tlr4 signaling, as well as dozens of previously unknown candidates that we validated. By measuring protein markers and mRNA profiles in DCs that are deficient in known or candidate genes, we classified the genes into three functional modules with distinct effects on the canonical responses to LPS and highlighted functions for the PAF complex and oligosaccharyltransferase (OST) complex. Our findings uncover new facets of innate immune circuits in primary cells and provide a genetic approach for dissection of mammalian cell circuits.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




References
-
- Beaudoin G, Jaffrin MY. Plasma filtration in Couette flow membrane devices. Artificial organs. 1989;13:43–51. - PubMed
-
- Bochenek G, Hasler R, El Mokhtari NE, Konig IR, Loos BG, Jepsen S, Rosenstiel P, Schreiber S, Schaefer AS. The large non-coding RNA ANRIL, which is associated with atherosclerosis, periodontitis and several forms of cancer, regulates ADIPOR1, VAMP3 and C11ORF10. Human molecular genetics. 2013;22:4516–4527. - PubMed
-
- Boehringer N, Hagens G, Songeon F, Isler P, Nicod LP. Differential regulation of tumor necrosing factor-alpha (TNF-alpha) and interleukin-10 (IL-10) secretion by protein kinase and phosphatase inhibitors in human alveolar macrophages. European cytokine network. 1999;10:211–218. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

