Regeneration of Sensory Hair Cells Requires Localized Interactions between the Notch and Wnt Pathways
- PMID: 26190147
- PMCID: PMC4557215
- DOI: 10.1016/j.devcel.2015.05.025
Regeneration of Sensory Hair Cells Requires Localized Interactions between the Notch and Wnt Pathways
Abstract
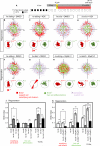
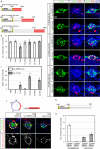
In vertebrates, mechano-electrical transduction of sound is accomplished by sensory hair cells. Whereas mammalian hair cells are not replaced when lost, in fish they constantly renew and regenerate after injury. In vivo tracking and cell fate analyses of all dividing cells during lateral line hair cell regeneration revealed that support and hair cell progenitors localize to distinct tissue compartments. Importantly, we find that the balance between self-renewal and differentiation in these compartments is controlled by spatially restricted Notch signaling and its inhibition of Wnt-induced proliferation. The ability to simultaneously study and manipulate individual cell behaviors and multiple pathways in vivo transforms the lateral line into a powerful paradigm to mechanistically dissect sensory organ regeneration. The striking similarities to other vertebrate stem cell compartments uniquely place zebrafish to help elucidate why mammals possess such low capacity to regenerate hair cells.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures






References
-
- Chen P, Zindy F, Abdala C, Liu F, Li X, Roussel MF, Segil N. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat Cell Biol. 2003;5:422–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

