Microfluidic device to control interstitial flow-mediated homotypic and heterotypic cellular communication
- PMID: 26190172
- PMCID: PMC4855298
- DOI: 10.1039/c5lc00507h
Microfluidic device to control interstitial flow-mediated homotypic and heterotypic cellular communication
Abstract
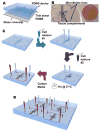
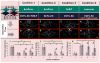
Tissue engineering can potentially recreate in vivo cellular microenvironments in vitro for an array of applications such as biological inquiry and drug discovery. However, the majority of current in vitro systems still neglect many biological, chemical, and mechanical cues that are known to impact cellular functions such as proliferation, migration, and differentiation. To address this gap, we have developed a novel microfluidic device that precisely controls the spatial and temporal interactions between adjacent three-dimensional cellular environments. The device consists of four interconnected microtissue compartments (~0.1 mm(3)) arranged in a square. The top and bottom pairs of compartments can be sequentially loaded with discrete cellularized hydrogels creating the opportunity to investigate homotypic (left to right or x-direction) and heterotypic (top to bottom or y-direction) cell-cell communication. A controlled hydrostatic pressure difference across the tissue compartments in both x and y direction induces interstitial flow and modulates communication via soluble factors. To validate the biological significance of this novel platform, we examined the role of stromal cells in the process of vasculogenesis. Our device confirms previous observations that soluble mediators derived from normal human lung fibroblasts (NHLFs) are necessary to form a vascular network derived from endothelial colony forming cell-derived endothelial cells (ECFC-ECs). We conclude that this platform could be used to study important physiological and pathological processes that rely on homotypic and heterotypic cell-cell communication.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

