Gαi/o-dependent Ca(2+) mobilization and Gαq-dependent PKCα regulation of Ca(2+)-sensing receptor-mediated responses in N18TG2 neuroblastoma cells
- PMID: 26190181
- PMCID: PMC4641771
- DOI: 10.1016/j.neuint.2015.07.008
Gαi/o-dependent Ca(2+) mobilization and Gαq-dependent PKCα regulation of Ca(2+)-sensing receptor-mediated responses in N18TG2 neuroblastoma cells
Abstract
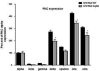
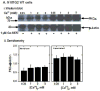
A functional Ca(2+)-sensing receptor (CaS) is expressed endogenously in mouse N18TG2 neuroblastoma cells, and sequence analysis of the cDNA indicates high homology with both rat and human parathyroid CaS cDNAs. The CaS in N18TG2 cells appears as a single immunoreactive protein band at about 150 kDa on Western blots, consistent with native CaS from dorsal root ganglia. Both wild type (WT) and Gαq antisense knock-down (KD) cells responded to Ca(2+) and calindol, a positive allosteric modulator of the CaS, with a transient increase in intracellular Ca(2+) concentration ([Ca(2+)]i), which was larger in the Gαq KD cells. Stimulation with 1 mM extracellular Ca(2+) (Ca(2+)e) increased [Ca(2+)]i in N18TG2 Gαq KD compared to WT cells. Ca(2+) mobilization was dependent on pertussis toxin-sensitive Gαi/o proteins and reduced by 30 μM 2-amino-ethyldiphenyl borate and 50 μM nifedipine to the same plateau levels in both cell types. Membrane-associated PKCα and p-PKCα increased with increasing [Ca(2+)]e in WT cells, but decreased in Gαq KD cells. Treatment of cells with 1 μM Gӧ 6976, a Ca(2+)-specific PKC inhibitor reduced Ca(2+) mobilization and membrane-associated PKCα and p-PKCα in both cell types. The results indicate that the CaS-mediated increase in [Ca(2+)]i in N18TG2 cells is dependent on Gαi/o proteins via inositol-1,4,5-triphosphate (IP3) channels and store-operated Ca(2+) entry channels, whereas modulation of CaS responses involving PKCα phosphorylation and translocation to the plasma membrane occurs via a Gαq mechanism.
Keywords: Ca(2+) mobilization; CaS responses; Gα(i/o); Gα(q) antisense knock-down; N18TG2 cells; Neuronal CaS; Protein kinase C.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
















References
-
- Awumey EM, Howlett AC, Putney JW, Jr, Diz DI, Bukoski RD. Ca(2+) mobilization through dorsal root ganglion Ca(2+)-sensing receptor stably expressed in HEK293 cells. Am J Physiol Cell Physiol. 2007;292:C1895–1905. - PubMed
-
- Bai M, Trivedi S, Lane CR, Yang Y, Quinn SJ, Brown EM. Protein kinase C phosphorylation of threonine at position 888 in Ca2+o-sensing receptor (CaR) inhibits coupling to Ca2+ store release. J Biol Chem. 1998;273:21267–21275. - PubMed
-
- Berridge MJ. Calcium signal transduction and cellular control mechanisms. Biochim Biophys Acta. 2004;1742:3–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

