Repeated Injections of IL-2 Break Renal Allograft Tolerance Induced via Mixed Hematopoietic Chimerism in Monkeys
- PMID: 26190648
- PMCID: PMC4654979
- DOI: 10.1111/ajt.13382
Repeated Injections of IL-2 Break Renal Allograft Tolerance Induced via Mixed Hematopoietic Chimerism in Monkeys
Abstract
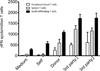
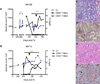
Tolerance of allografts achieved in mice via stable mixed hematopoietic chimerism relies essentially on continuous elimination of developing alloreactive T cells in the thymus (central deletion). Conversely, while only transient mixed chimerism is observed in nonhuman primates and patients, it is sufficient to ensure tolerance of kidney allografts. In this setting, it is likely that tolerance depends on peripheral regulatory mechanisms rather than thymic deletion. This implies that, in primates, upsetting the balance between inflammatory and regulatory alloimmunity could abolish tolerance and trigger the rejection of previously accepted renal allografts. In this study, six monkeys that were treated with a mixed chimerism protocol and had accepted a kidney allograft for periods of 1-10 years after withdrawal of immunosuppression received subcutaneous injections of IL-2 cytokine (0.6-3 × 10(6) IU/m(2) ). This resulted in rapid rejection of previously tolerated renal transplants and was associated with an expansion and reactivation of alloreactive pro-inflammatory memory T cells in the host's lymphoid organs and in the graft. This phenomenon was prevented by anti-CD8 antibody treatment. Finally, this process was reversible in that cessation of IL-2 administration aborted the rejection process and restored normal kidney graft function.
Keywords: animal models: nonhuman primate; basic (laboratory) research/science; bone marrow/hematopoietic stem cell transplantation; immunosuppression/immune modulation; kidney (allograft) function/dysfunction; kidney transplantation/nephrology; tolerance: chimerism; tolerance: mechanisms; translational research/science.
© Copyright 2015 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures






Similar articles
-
Use of CTLA4Ig for induction of mixed chimerism and renal allograft tolerance in nonhuman primates.Am J Transplant. 2014 Dec;14(12):2704-12. doi: 10.1111/ajt.12936. Epub 2014 Nov 13. Am J Transplant. 2014. PMID: 25394378 Free PMC article.
-
Transient-mixed Chimerism With Nonmyeloablative Conditioning Does Not Induce Liver Allograft Tolerance in Nonhuman Primates.Transplantation. 2020 Aug;104(8):1580-1590. doi: 10.1097/TP.0000000000003263. Transplantation. 2020. PMID: 32732835 Free PMC article.
-
Effect of Ex Vivo-Expanded Recipient Regulatory T Cells on Hematopoietic Chimerism and Kidney Allograft Tolerance Across MHC Barriers in Cynomolgus Macaques.Transplantation. 2017 Feb;101(2):274-283. doi: 10.1097/TP.0000000000001559. Transplantation. 2017. PMID: 27846155 Free PMC article.
-
Chimerism-Based Tolerance to Kidney Allografts in Humans: Novel Insights and Future Perspectives.Front Immunol. 2022 Jan 5;12:791725. doi: 10.3389/fimmu.2021.791725. eCollection 2021. Front Immunol. 2022. PMID: 35069574 Free PMC article. Review.
-
Preclinical and clinical studies for transplant tolerance via the mixed chimerism approach.Hum Immunol. 2018 May;79(5):258-265. doi: 10.1016/j.humimm.2017.11.008. Epub 2017 Nov 22. Hum Immunol. 2018. PMID: 29175110 Free PMC article. Review.
Cited by
-
Tracking of TCR-Transgenic T Cells Reveals That Multiple Mechanisms Maintain Cardiac Transplant Tolerance in Mice.Am J Transplant. 2016 Oct;16(10):2854-2864. doi: 10.1111/ajt.13814. Epub 2016 May 5. Am J Transplant. 2016. PMID: 27091509 Free PMC article.
-
Assessment of the Relationship Between Inflammation and Glomerular Filtration Rate.Can J Kidney Health Dis. 2023 Jan 19;10:20543581221132748. doi: 10.1177/20543581221132748. eCollection 2023. Can J Kidney Health Dis. 2023. PMID: 36700057 Free PMC article.
-
Immune Tolerance Therapy: A New Method for Treatment of Traumatic Brain Injury.Chin Med J (Engl). 2018 Aug 20;131(16):1990-1998. doi: 10.4103/0366-6999.238147. Chin Med J (Engl). 2018. PMID: 30082532 Free PMC article. Review.
-
Transplantation Tolerance through Hematopoietic Chimerism: Progress and Challenges for Clinical Translation.Front Immunol. 2017 Dec 22;8:1762. doi: 10.3389/fimmu.2017.01762. eCollection 2017. Front Immunol. 2017. PMID: 29312303 Free PMC article. Review.
-
Transplantation tolerance after allograft rejection.Curr Opin Organ Transplant. 2017 Feb;22(1):64-70. doi: 10.1097/MOT.0000000000000374. Curr Opin Organ Transplant. 2017. PMID: 27898463 Free PMC article. Review.
References
-
- Sykes M, Sachs DH. Mixed allogeneic chimerism as an approach to transplantation tolerance. Immunol Today. 1988;9:23–27. - PubMed
-
- Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J Immunol. 1994;153:1087–1098. - PubMed
-
- Khan A, Tomita Y, Sykes M. Thymic dependence of loss of tolerance in mixed allogeneic bone marrow chimeras after depletion of donor antigen. Peripheral mechanisms do not contribute to maintenance of tolerance. Transplantation. 1996;62:380–387. - PubMed
-
- Sharabi Y, Abraham VS, Sykes M, Sachs DH. Mixed allogeneic chimeras prepared by a nonmyeloablative regimen: Requirement for chimerism to maintain tolerance. Bone Marrow Transplant. 1992;9:191–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

