Metabolic Regulation of Histone Acetyltransferases by Endogenous Acyl-CoA Cofactors
- PMID: 26190825
- PMCID: PMC4546520
- DOI: 10.1016/j.chembiol.2015.06.015
Metabolic Regulation of Histone Acetyltransferases by Endogenous Acyl-CoA Cofactors
Abstract
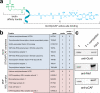
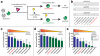
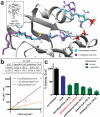
The finding that chromatin modifications are sensitive to changes in cellular cofactor levels potentially links altered tumor cell metabolism and gene expression. However, the specific enzymes and metabolites that connect these two processes remain obscure. Characterizing these metabolic-epigenetic axes is critical to understanding how metabolism supports signaling in cancer, and developing therapeutic strategies to disrupt this process. Here, we describe a chemical approach to define the metabolic regulation of lysine acetyltransferase (KAT) enzymes. Using a novel chemoproteomic probe, we identify a previously unreported interaction between palmitoyl coenzyme A (palmitoyl-CoA) and KAT enzymes. Further analysis reveals that palmitoyl-CoA is a potent inhibitor of KAT activity and that fatty acyl-CoA precursors reduce cellular histone acetylation levels. These studies implicate fatty acyl-CoAs as endogenous regulators of histone acetylation, and suggest novel strategies for the investigation and metabolic modulation of epigenetic signaling.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


References
-
- Agius L, Wright PD, Alberti KG. Carnitine acyltransferases and acyl-CoA hydrolases in human and rat liver. Clin Sci (Lond) 1987;73:3–10. - PubMed
-
- Becher I, Savitski MM, Savitski MF, Hopf C, Bantscheff M, Drewes G. Affinity profiling of the cellular kinome for the nucleotide cofactors ATP, ADP, and GTP. ACS Chem Biol. 2013;8:599–607. - PubMed
-
- Chase JF, Middleton B, Tubbs PK. A coenzyme A analogue, desulphocoA; preparation and effects on various enzymes. Biochem. Biophys. Res. Commun. 1966;23:208–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

