Hormonal status and age differentially affect tolerance to the disruptive effects of delta-9-tetrahydrocannabinol (Δ(9)-THC) on learning in female rats
- PMID: 26191005
- PMCID: PMC4488627
- DOI: 10.3389/fphar.2015.00133
Hormonal status and age differentially affect tolerance to the disruptive effects of delta-9-tetrahydrocannabinol (Δ(9)-THC) on learning in female rats
Abstract
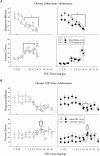
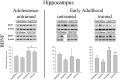
The effects of hormone status and age on the development of tolerance to Δ(9)-THC were assessed in sham-operated (intact) or ovariectomized (OVX) female rats that received either intraperitoneal saline or 5.6 mg/kg of Δ(9)-THC daily from postnatal day (PD) 75-180 (early adulthood onward) or PD 35-140 (adolescence onward). During this time, the four groups for each age (i.e., intact/saline, intact/THC, OVX/saline, and OVX/THC) were trained in a learning and performance procedure and dose-effect curves were established for Δ(9)-THC (0.56-56 mg/kg) and the cannabinoid type-1 receptor (CB1R) antagonist rimonabant (0.32-10 mg/kg). Despite the persistence of small rate-decreasing and error-increasing effects in intact and OVX females from both ages during chronic Δ(9)-THC, all of the Δ(9)-THC groups developed tolerance. However, the magnitude of tolerance, as well as the effect of hormone status, varied with the age at which chronic Δ(9)-THC was initiated. There was no evidence of dependence in any of the groups. Hippocampal protein expression of CB1R, AHA1 (a co-chaperone of CB1R) and HSP90β (a molecular chaperone modulated by AHA-1) was affected more by OVX than chronic Δ(9)-THC; striatal protein expression was not consistently affected by either manipulation. Hippocampal brain-derived neurotrophic factor expression varied with age, hormone status, and chronic treatment. Thus, hormonal status differentially affects the development of tolerance to the disruptive effects of delta-9-tetrahydrocannabinol (Δ(9)-THC) on learning and performance behavior in adolescent, but not adult, female rats. These factors and their interactions also differentially affect cannabinoid signaling proteins in the hippocampus and striatum, and ultimately, neural plasticity.
Keywords: age of initiation; cannabinoid receptors; chronic Δ9-tetrahydrocannabinol; learning; ovariectomy; rat.
Figures





Similar articles
-
Chronic administration during early adulthood does not alter the hormonally-dependent disruptive effects of delta-9-tetrahydrocannabinol (Δ9-THC) on complex behavior in female rats.Pharmacol Biochem Behav. 2014 Feb;117:118-27. doi: 10.1016/j.pbb.2013.12.014. Epub 2013 Dec 18. Pharmacol Biochem Behav. 2014. PMID: 24361784 Free PMC article.
-
Ovarian hormones and chronic administration during adolescence modify the discriminative stimulus effects of delta-9-tetrahydrocannabinol (Δ⁹-THC) in adult female rats.Pharmacol Biochem Behav. 2012 Sep;102(3):442-9. doi: 10.1016/j.pbb.2012.06.008. Epub 2012 Jun 15. Pharmacol Biochem Behav. 2012. PMID: 22705493 Free PMC article.
-
Long-term behavioral and pharmacodynamic effects of delta-9-tetrahydrocannabinol in female rats depend on ovarian hormone status.Addict Biol. 2011 Jan;16(1):64-81. doi: 10.1111/j.1369-1600.2010.00227.x. Addict Biol. 2011. PMID: 21158010 Free PMC article.
-
Sex and age specific effects of delta-9-tetrahydrocannabinol during the periadolescent period in the rat: The unique susceptibility of the prepubescent animal.Neurotoxicol Teratol. 2016 Nov-Dec;58:88-100. doi: 10.1016/j.ntt.2016.02.005. Epub 2016 Feb 16. Neurotoxicol Teratol. 2016. PMID: 26898326 Review.
-
Escalating low-dose Δ9 -tetrahydrocannabinol exposure during adolescence induces differential behavioral and neurochemical effects in male and female adult rats.Eur J Neurosci. 2020 Jul;52(1):2681-2693. doi: 10.1111/ejn.14598. Epub 2019 Nov 9. Eur J Neurosci. 2020. PMID: 31626712 Review.
Cited by
-
Chronic Δ9-Tetrahydrocannabinol during Adolescence Differentially Modulates Striatal CB1 Receptor Expression and the Acute and Chronic Effects on Learning in Adult Rats.J Pharmacol Exp Ther. 2016 Jan;356(1):20-31. doi: 10.1124/jpet.115.227181. Epub 2015 Oct 13. J Pharmacol Exp Ther. 2016. PMID: 26462539 Free PMC article.
-
Tolerance to hypothermic and antinoceptive effects of ∆9-tetrahydrocannabinol (THC) vapor inhalation in rats.Pharmacol Biochem Behav. 2018 Sep;172:33-38. doi: 10.1016/j.pbb.2018.07.007. Epub 2018 Jul 19. Pharmacol Biochem Behav. 2018. PMID: 30031028 Free PMC article.
-
Effect of Cannabis on Memory Consolidation, Learning and Retrieval and Its Current Legal Status in India: A Review.Biomolecules. 2023 Jan 12;13(1):162. doi: 10.3390/biom13010162. Biomolecules. 2023. PMID: 36671547 Free PMC article. Review.
-
Δ9-Tetrahydrocannabinol discrimination: Effects of route of administration in rats.Drug Alcohol Depend. 2021 Aug 1;225:108827. doi: 10.1016/j.drugalcdep.2021.108827. Epub 2021 Jun 23. Drug Alcohol Depend. 2021. PMID: 34186444 Free PMC article.
-
Multigenerational consequences of early-life cannabinoid exposure in zebrafish.Toxicol Appl Pharmacol. 2019 Feb 1;364:133-143. doi: 10.1016/j.taap.2018.12.021. Epub 2018 Dec 27. Toxicol Appl Pharmacol. 2019. PMID: 30594692 Free PMC article.
References
-
- Aceto M. D., Scates S. M., Lowe J. A., Martin B. R. (1996). Dependence on delta 9-tetrahydrocannabinol: studies on precipitated and abrupt withdrawal. J. Pharmacol. Exp. Ther. 278 1290–1295. - PubMed
-
- Ali S. F., Newport G. D., Scallet A. C., Gee K. W., Paule M. G., Brown R. M., et al. (1989). Effects of chronic delta-9-tetrahydrocannabinol (THC) administration on neurotransmitter concentrations and receptor binding in the rat brain. Neurotoxicology 10 491–500. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

