Composite Ni/NiO-Cr2O3 Catalyst for Alkaline Hydrogen Evolution Reaction
- PMID: 26191118
- PMCID: PMC4501498
- DOI: 10.1021/jp512311c
Composite Ni/NiO-Cr2O3 Catalyst for Alkaline Hydrogen Evolution Reaction
Abstract
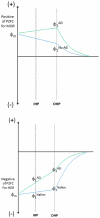
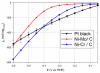
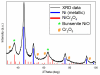
We report a Ni-Cr/C electrocatalyst with unprecedented mass-activity for the hydrogen evolution reaction (HER) in alkaline electrolyte. The HER kinetics of numerous binary and ternary Ni-alloys and composite Ni/metal-oxide/C samples were evaluated in aqueous 0.1 M KOH electrolyte. The highest HER mass-activity was observed for Ni-Cr materials which exhibit metallic Ni as well as NiO x and Cr2O3 phases as determined by X-ray diffraction (XRD) and X-ray absorption spectroscopy (XAS) analysis. The onset of the HER is significantly improved compared to numerous binary and ternary Ni-alloys, including Ni-Mo materials. It is likely that at adjacent Ni/NiO x sites, the oxide acts as a sink for OHads, while the metallic Ni acts as a sink for the Hads intermediate of the HER, thus minimizing the high activation energy of hydrogen evolution via water reduction. This is confirmed by in situ XAS studies that show that the synergistic HER enhancement is due to NiO x content and that the Cr2O3 appears to stabilize the composite NiO x component under HER conditions (where NiO x would typically be reduced to metallic Ni0). Furthermore, in contrast to Pt, the Ni(O x )/Cr2O3 catalyst appears resistant to poisoning by the anion exchange ionomer (AEI), a serious consideration when applied to an anionic polymer electrolyte interface. Furthermore, we report a detailed model of the double layer interface which helps explain the observed ensemble effect in the presence of AEI.
Figures







References
-
- Carmo M, Fritz DL, Mergel J, Stolten D. A Comprehensive Review on Pem Water Electrolysis. Int. J. Hydrogen Energy. 2013;38:4901–4934.
-
- Kriston A, Xie T, Popov BN. Impact of Ultra-Low Platinum Loading on Mass Activity and Mass Transport in H2-Oxygen and H2- Air Pem Fuel Cells. Electrochim. Acta. 2014;121:116–127.
-
- Urian RC, Gullá AF, Mukerjee S. Electrocatalysis of Reformate Tolerance in Proton Exchange Membranes Fuel Cells: Part I. J. Electroanal. Chem. 2003:554–555. 307–324.
-
- Mukerjee S, Urian RC, Lee SJ, Ticianelli EA, McBreen J. Electrocatalysis of Co Tolerance by Carbon-Supported Ptmo Electro-catalysts in PEMFCs. J. Electrochem. Soc. 2004;151:A1094–A1103.
-
- Ehteshami SMM, Jia Q, Halder A, Chan SH, Mukerjee S. The Role of Electronic Properties of Pt and Pt Alloys for Enhanced Reformate Electro-Oxidation in Polymer Electrolyte Membrane Fuel Cells. Electrochim. Acta. 2013;107:155–163.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
