Fragment-Based Discovery of Potent and Selective DDR1/2 Inhibitors
- PMID: 26191369
- PMCID: PMC4499826
- DOI: 10.1021/acsmedchemlett.5b00143
Fragment-Based Discovery of Potent and Selective DDR1/2 Inhibitors
Abstract
The DDR1 and DDR2 receptor tyrosine kinases are activated by extracellular collagen and have been implicated in a number of human diseases including cancer. We performed a fragment-based screen against DDR1 and identified fragments that bound either at the hinge or in the back pocket associated with the DFG-out conformation of the kinase. Modeling based on crystal structures of potent kinase inhibitors facilitated the "back-to-front" design of potent DDR1/2 inhibitors that incorporated one of the DFG-out fragments. Further optimization led to low nanomolar, orally bioavailable inhibitors that were selective for DDR1 and DDR2. The inhibitors were shown to potently inhibit DDR2 activity in cells but in contrast to unselective inhibitors such as dasatinib, they did not inhibit proliferation of mutant DDR2 lung SCC cell lines.
Keywords: back to front kinase design; discoidin domain receptor; fragment-based drug design.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
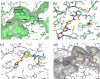
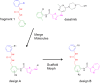
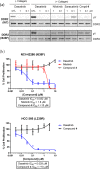
References
-
- Shrivastava A.; Radziejewski C.; Campbell E.; Kovac L.; McGlynn M.; Ryan T. E.; Davis S.; Goldfarb M. P.; Glass D. J.; Lemke G.; Yancopoulos G. D. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol. Cell 1997, 1, 25–34. - PubMed
-
- Leitinger B. Transmembrane collagen receptors. Annu. Rev. Cell Dev. Biol. 2011, 27, 265–290. - PubMed
-
- Vogel W. F.; Abdulhussein R.; Ford C. E. Sensing extracellular matrix: an update on discoidin domain receptor function. Cell. Signal. 2006, 18, 1108–1116. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

