The Mitochondrial Peptidase Pitrilysin Degrades Islet Amyloid Polypeptide in Beta-Cells
- PMID: 26191799
- PMCID: PMC4507941
- DOI: 10.1371/journal.pone.0133263
The Mitochondrial Peptidase Pitrilysin Degrades Islet Amyloid Polypeptide in Beta-Cells
Abstract
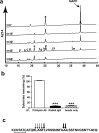
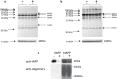
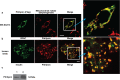
Amyloid formation and mitochondrial dysfunction are characteristics of type 2 diabetes. The major peptide constituent of the amyloid deposits in type 2 diabetes is islet amyloid polypeptide (IAPP). In this study, we found that pitrilysin, a zinc metallopeptidase of the inverzincin family, degrades monomeric, but not oligomeric, islet amyloid polypeptide in vitro. In insulinoma cells when pitrilysin expression was decreased to 5% of normal levels, there was a 60% increase in islet amyloid polypeptide-induced apoptosis. In contrast, overexpression of pitrilysin protects insulinoma cells from human islet amyloid polypeptide-induced apoptosis. Since pitrilysin is a mitochondrial protein, we used immunofluorescence staining of pancreases from human IAPP transgenic mice and Western blot analysis of IAPP in isolated mitochondria from insulinoma cells to provide evidence for a putative intramitochondrial pool of IAPP. These results suggest that pitrilysin regulates islet amyloid polypeptide in beta cells and suggest the presence of an intramitochondrial pool of islet amyloid polypeptide involved in beta-cell apoptosis.
Conflict of interest statement
Figures




Similar articles
-
Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway.Am J Pathol. 2010 Feb;176(2):861-9. doi: 10.2353/ajpath.2010.090532. Epub 2009 Dec 30. Am J Pathol. 2010. PMID: 20042670 Free PMC article.
-
Functional proteasome complex is required for turnover of islet amyloid polypeptide in pancreatic β-cells.J Biol Chem. 2018 Sep 14;293(37):14210-14223. doi: 10.1074/jbc.RA118.002414. Epub 2018 Jul 16. J Biol Chem. 2018. PMID: 30012886 Free PMC article.
-
Immunocytochemical staining for islet amyloid polypeptide in pancreatic endocrine tumors.Islets. 2011 Nov-Dec;3(6):344-51. doi: 10.4161/isl.3.6.17866. Epub 2011 Nov 1. Islets. 2011. PMID: 21983096 Free PMC article.
-
Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes.ILAR J. 2006;47(3):225-33. doi: 10.1093/ilar.47.3.225. ILAR J. 2006. PMID: 16804197 Review.
-
Causative factors for formation of toxic islet amyloid polypeptide oligomer in type 2 diabetes mellitus.Clin Interv Aging. 2015 Nov 19;10:1873-9. doi: 10.2147/CIA.S95297. eCollection 2015. Clin Interv Aging. 2015. PMID: 26604727 Free PMC article. Review.
Cited by
-
The association between renal accumulation of pancreatic amyloid-forming amylin and renal hypoxia.Front Endocrinol (Lausanne). 2023 Feb 16;14:1104662. doi: 10.3389/fendo.2023.1104662. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36875454 Free PMC article. Review.
References
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–10. . - PubMed
-
- Johnson KH, O'Brien TD, Betsholtz C, Westermark P. Islet amyloid polypeptide: mechanisms of amyloidogenesis in the pancreatic islets and potential roles in diabetes mellitus. Lab Invest. 1992;66(5):522–35. . - PubMed
-
- Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48(2):241–53. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

