Ubc9 Impairs Activation of the Brown Fat Energy Metabolism Program in Human White Adipocytes
- PMID: 26192107
- PMCID: PMC4552434
- DOI: 10.1210/me.2015-1084
Ubc9 Impairs Activation of the Brown Fat Energy Metabolism Program in Human White Adipocytes
Abstract
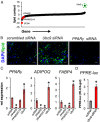
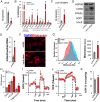
Insulin resistance and type 2 diabetes mellitus (T2DM) result from an inability to efficiently store and catabolize surplus energy in adipose tissue. Subcutaneous adipocytes protect against insulin resistance and T2DM by coupling differentiation with the induction of brown fat gene programs for efficient energy metabolism. Mechanisms that disrupt these programs in adipocytes are currently poorly defined, but represent therapeutic targets for the treatment of T2DM. To gain insight into these mechanisms, we performed a high-throughput microscopy screen that identified ubiquitin carrier protein 9 (Ubc9) as a negative regulator of energy storage in human sc adipocytes. Ubc9 depletion enhanced energy storage and induced the brown fat gene program in human sc adipocytes. Induction of adipocyte differentiation resulted in decreased Ubc9 expression commensurate with increased brown fat gene expression. Thiazolidinedione treatment reduced the interaction between Ubc9 and peroxisome proliferator-activated receptor (PPAR)γ, suggesting a mechanism by which Ubc9 represses PPARγ activity. In support of this hypothesis, Ubc9 overexpression remodeled energy metabolism in human sc adipocytes by selectively inhibiting brown adipocyte-specific function. Further, Ubc9 overexpression decreased uncoupling protein 1 expression by disrupting PPARγ binding at a critical uncoupling protein 1 enhancer region. Last, Ubc9 is significantly elevated in sc adipose tissue isolated from mouse models of insulin resistance as well as diabetic and insulin-resistant humans. Taken together, our findings demonstrate a critical role for Ubc9 in the regulation of sc adipocyte energy homeostasis.
Figures



Similar articles
-
Mitochondrial Activity in Human White Adipocytes Is Regulated by the Ubiquitin Carrier Protein 9/microRNA-30a Axis.J Biol Chem. 2016 Nov 18;291(47):24747-24755. doi: 10.1074/jbc.M116.749408. Epub 2016 Oct 10. J Biol Chem. 2016. PMID: 27758866 Free PMC article.
-
Peroxisome proliferator-activated receptors-α and -γ, and cAMP-mediated pathways, control retinol-binding protein-4 gene expression in brown adipose tissue.Endocrinology. 2012 Mar;153(3):1162-73. doi: 10.1210/en.2011-1367. Epub 2012 Jan 17. Endocrinology. 2012. PMID: 22253419
-
Role of UBC9 in the regulation of the adipogenic program in 3T3-L1 adipocytes.Endocrinology. 2010 Nov;151(11):5255-66. doi: 10.1210/en.2010-0417. Epub 2010 Sep 29. Endocrinology. 2010. PMID: 20881252
-
Role of PPARg2 transcription factor in thiazolidinedione-induced insulin sensitization.J Pharm Pharmacol. 2012 Feb;64(2):161-71. doi: 10.1111/j.2042-7158.2011.01366.x. Epub 2011 Oct 13. J Pharm Pharmacol. 2012. PMID: 22221092 Review.
-
Brown vs white adipocytes: the PPARgamma coregulator story.FEBS Lett. 2010 Aug 4;584(15):3250-9. doi: 10.1016/j.febslet.2010.06.035. Epub 2010 Jun 30. FEBS Lett. 2010. PMID: 20600006 Review.
Cited by
-
miR-30a Remodels Subcutaneous Adipose Tissue Inflammation to Improve Insulin Sensitivity in Obesity.Diabetes. 2018 Dec;67(12):2541-2553. doi: 10.2337/db17-1378. Epub 2018 Jul 12. Diabetes. 2018. PMID: 30002134 Free PMC article.
-
miR-30a targets gene networks that promote browning of human and mouse adipocytes.Am J Physiol Endocrinol Metab. 2020 Oct 1;319(4):E667-E677. doi: 10.1152/ajpendo.00045.2020. Epub 2020 Aug 17. Am J Physiol Endocrinol Metab. 2020. PMID: 32799658 Free PMC article.
-
A Provocative Molecular Link between Mammographic Density and BRCA1-loss associated TNBC.Int J Hum Genet Genet Disord. 2019;1(1):1-8. doi: 10.18689/ijhg-1000101. Epub 2019 Jun 21. Int J Hum Genet Genet Disord. 2019. PMID: 33860286 Free PMC article.
-
Evidence for the Neuronal Expression and Secretion of Adiponectin.Cells. 2022 Sep 1;11(17):2725. doi: 10.3390/cells11172725. Cells. 2022. PMID: 36078135 Free PMC article.
-
Tissue adaptation to metabolic stress: insights from SUMOylation.Front Endocrinol (Lausanne). 2024 Nov 11;15:1434338. doi: 10.3389/fendo.2024.1434338. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39588331 Free PMC article. Review.
References
-
- Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep. 2010;10(4):306–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01GM033976/GM/NIGMS NIH HHS/United States
- R01 ES023206/ES/NIEHS NIH HHS/United States
- RC2 ES018789/ES/NIEHS NIH HHS/United States
- P30-DK079638/DK/NIDDK NIH HHS/United States
- U54 HD-007495/HD/NICHD NIH HHS/United States
- 1RC2ES018789/ES/NIEHS NIH HHS/United States
- P30 DK-56338/DK/NIDDK NIH HHS/United States
- 1R01ES023206/ES/NIEHS NIH HHS/United States
- U54 HD007495/HD/NICHD NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- P30 DK056338/DK/NIDDK NIH HHS/United States
- P30 DK079638/DK/NIDDK NIH HHS/United States
- K01 DK096093/DK/NIDDK NIH HHS/United States
- R01 GM033976/GM/NIGMS NIH HHS/United States
- K01DK096093/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

