Electronic, Dielectric, and Plasmonic Properties of Two-Dimensional Electride Materials X2N (X=Ca, Sr): A First-Principles Study
- PMID: 26192245
- PMCID: PMC5387395
- DOI: 10.1038/srep12285
Electronic, Dielectric, and Plasmonic Properties of Two-Dimensional Electride Materials X2N (X=Ca, Sr): A First-Principles Study
Abstract
Based on first-principles calculations, we systematically study the electronic, dielectric, and plasmonic properties of two-dimensional (2D) electride materials X2N (X=Ca, Sr). We show that both Ca2N and Sr2N are stable down to monolayer thickness. For thicknesses larger than 1-monolayer (1-ML), there are 2D anionic electron layers confined in the regions between the [X2N](+) layers. These electron layers are strongly trapped and have weak coupling between each other. As a result, for the thickness dependence of many properties such as the surface energy, work function, and dielectric function, the most dramatic change occurs when going from 1-ML to 2-ML. For both bulk and few-layer Ca2N and Sr2N, the in-plane and out-of-plane real components of their dielectric functions have different signs in an extended frequency range covering the near infrared, indicating their potential applications as indefinite media. We find that bulk Ca2N and Sr2N could support surface plasmon modes in the near infrared range. Moreover, tightly-bounded plasmon modes could exist in their few-layer structures. These modes have significantly shorter wavelengths (few tens of nanometers) compared with that of conventional noble metal materials, suggesting their great potential for plasmonic devices with much smaller dimensions.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

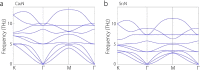
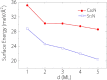






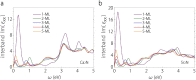


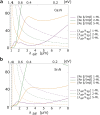
References
-
- Dye J. L. Electrons as Anions. Science 301, 607–608 (2003). - PubMed
-
- Dye J. L. Electrides: early examples of quantum confinement. Acc.Chem.Res. 42, 1564–1572 (2009). - PubMed
-
- Ellaboudy A., Dye J. L. & Smith P. B. Cesium 18-Crown-6 Compounds. A Crystalline Ceside and a Crystalline Electride. J. Am. Chem. Soc. 105, 6490–6491 (1983).
-
- Huang R. H., Faber M. K., Moeggenborg K. J., Ward D. L. & Dye J. L. Structure of K+ (cryptand[2.2.2J) electride and evidence for trapped electron pairs. Nature 331, 599–601 (1988).
-
- Dye J. L. Electrides: Ionic Salts with Electrons as the Anions. Science 247, 663–668 (1990). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

