Hepatic progenitor cells of biliary origin with liver repopulation capacity
- PMID: 26192438
- PMCID: PMC4612439
- DOI: 10.1038/ncb3203
Hepatic progenitor cells of biliary origin with liver repopulation capacity
Abstract
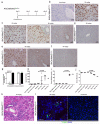
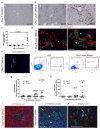
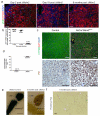
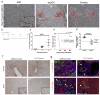
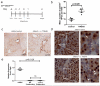
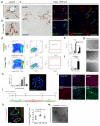
Hepatocytes and cholangiocytes self-renew following liver injury. Following severe injury hepatocytes are increasingly senescent, but whether hepatic progenitor cells (HPCs) then contribute to liver regeneration is unclear. Here, we describe a mouse model where the E3 ubiquitin ligase Mdm2 is inducibly deleted in more than 98% of hepatocytes, causing apoptosis, necrosis and senescence with nearly all hepatocytes expressing p21. This results in florid HPC activation, which is necessary for survival, followed by complete, functional liver reconstitution. HPCs isolated from genetically normal mice, using cell surface markers, were highly expandable and phenotypically stable in vitro. These HPCs were transplanted into adult mouse livers where hepatocyte Mdm2 was repeatedly deleted, creating a non-competitive repopulation assay. Transplanted HPCs contributed significantly to restoration of liver parenchyma, regenerating hepatocytes and biliary epithelia, highlighting their in vivo lineage potency. HPCs are therefore a potential future alternative to hepatocyte or liver transplantation for liver disease.
Figures


Comment in
-
Regenerative medicine: Hepatic progenitor cells up their game in the therapeutic stakes.Nat Rev Gastroenterol Hepatol. 2015 Nov;12(11):610-1. doi: 10.1038/nrgastro.2015.168. Epub 2015 Oct 6. Nat Rev Gastroenterol Hepatol. 2015. PMID: 26441248
-
Chasing the facultative liver progenitor cell.Hepatology. 2016 Jul;64(1):297-300. doi: 10.1002/hep.28642. Epub 2016 May 27. Hepatology. 2016. PMID: 27178427 No abstract available.
References
-
- Horslen SP, Fox IJ. Hepatocyte transplantation. Transplantation. 2004;77:1481–1486. - PubMed
-
- Horslen SP, et al. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics. 2003;111:1262–1267. - PubMed
-
- Hay DC. Cadaveric hepatocytes repopulate diseased livers: life after death. Gastroenterology. 2010;139:729–731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

