The Causal Role of IL-4 and IL-13 in Schistosoma mansoni Pulmonary Hypertension
- PMID: 26192556
- PMCID: PMC4642207
- DOI: 10.1164/rccm.201410-1820OC
The Causal Role of IL-4 and IL-13 in Schistosoma mansoni Pulmonary Hypertension
Abstract
Rationale: The etiology of schistosomiasis-associated pulmonary arterial hypertension (PAH), a major cause of PAH worldwide, is poorly understood. Schistosoma mansoni exposure results in prototypical type-2 inflammation. Furthermore, transforming growth factor (TGF)-β signaling is required for experimental pulmonary hypertension (PH) caused by Schistosoma exposure.
Objectives: We hypothesized type-2 inflammation driven by IL-4 and IL-13 is necessary for Schistosoma-induced TGF-β-dependent vascular remodeling.
Methods: Wild-type, IL-4(-/-), IL-13(-/-), and IL-4(-/-)IL-13(-/-) mice (C57BL6/J background) were intraperitoneally sensitized and intravenously challenged with S. mansoni eggs to induce experimental PH. Right ventricular catheterization was then performed, followed by quantitative analysis of the lung tissue. Lung tissue from patients with schistosomiasis-associated and connective tissue disease-associated PAH was also systematically analyzed.
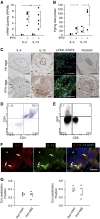
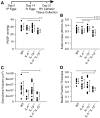
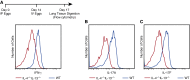
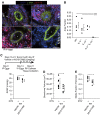
Measurements and main results: Mice with experimental Schistosoma-induced PH had evidence of increased IL-4 and IL-13 signaling. IL-4(-/-)IL-13(-/-) mice, but not single knockout IL-4(-/-) or IL-13(-/-) mice, were protected from Schistosoma-induced PH, with decreased right ventricular pressures, pulmonary vascular remodeling, and right ventricular hypertrophy. IL-4(-/-)IL-13(-/-) mice had less pulmonary vascular phospho-signal transducer and activator of transcription 6 (STAT6) and phospho-Smad2/3 activity, potentially caused by decreased TGF-β activation by macrophages. In vivo treatment with a STAT6 inhibitor and IL-4(-/-)IL-13(-/-) bone marrow transplantation also protected against Schistosoma-PH. Lung tissue from patients with schistosomiasis-associated and connective tissue disease-associated PAH had evidence of type-2 inflammation.
Conclusions: Combined IL-4 and IL-13 deficiency is required for protection against TGF-β-induced pulmonary vascular disease after Schistosoma exposure, and targeted inhibition of this pathway is a potential novel therapeutic approach for patients with schistosomiasis-associated PAH.
Keywords: Th2 cells; pulmonary hypertension; schistosomiasis; transforming growth factor-β.
Figures




Comment in
-
Spotlight on Inflammation in Pulmonary Hypertension.Am J Respir Crit Care Med. 2015 Oct 15;192(8):913-5. doi: 10.1164/rccm.201507-1426ED. Am J Respir Crit Care Med. 2015. PMID: 26469840 Free PMC article. No abstract available.
References
-
- Hovnanian A, Hoette S, Fernandes CJ, Jardim C, Souza R. Schistosomiasis associated pulmonary hypertension. Int J Clin Pract Suppl. 2010;(165):25–28. - PubMed
-
- Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25) Suppl:D34–D41. - PubMed
-
- Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31:163–176. - PubMed
-
- Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol. 2000;105:1063–1070. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

