Lipidoid Nanoparticles for siRNA Delivery to the Intestinal Epithelium: In Vitro Investigations in a Caco-2 Model
- PMID: 26192592
- PMCID: PMC4508104
- DOI: 10.1371/journal.pone.0133154
Lipidoid Nanoparticles for siRNA Delivery to the Intestinal Epithelium: In Vitro Investigations in a Caco-2 Model
Abstract
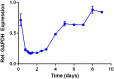
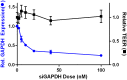
Short interfering ribonucleic acid (siRNA) therapeutics show promise for the treatment of intestinal diseases by specifically suppressing the expression of disease relevant proteins. Recently, a class of lipid-like materials termed "lipidoids" have been shown to potently deliver siRNA to the liver and immune cells. Here, we seek to establish the utility of lipidoid nanoparticles (LNPs) in the context of siRNA delivery to the intestinal epithelium. Initial studies demonstrated that the siRNA-loaded LNPs mediated potent, dose dependent, and durable gene silencing in Caco-2 intestinal epithelial cells, with a single 10 nM dose depressing GAPDH mRNA expression for one week. Transfection with siRNA-loaded LNPs did not induce significant cytotoxicity in Caco-2 cells or alter intestinal barrier function. Protein silencing was confirmed by Western blotting, with the lowest levels of GAPDH protein expression observed five days post-transfection. Together, these data underscore the potential of LNPs for the treatment of intestinal disorders.
Conflict of interest statement
Figures
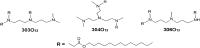




References
-
- Lautenschläger C, Schmidt C, Fischer D, Stallmach A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv Drug Deliv Rev. 2014;71(2013):58–76. - PubMed
-
- Jemal A, Freddie F, Center M, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):1, 33–64. - PubMed
-
- Talaei F, Atyabi F, Azhdarzadeh M, Dinarvand R, Saadatzadeh A. Overcoming therapeutic obstacles in inflammatory bowel diseases: A comprehensive review on novel drug delivery strategies. Eur J Pharm Sci. Elsevier B.V.; 2013;49(4):712–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

