Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection
- PMID: 26192873
- PMCID: PMC4569751
- DOI: 10.1056/NEJMoa1506816
Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection
Abstract
Background: Data from randomized trials are lacking on the benefits and risks of initiating antiretroviral therapy in patients with asymptomatic human immunodeficiency virus (HIV) infection who have a CD4+ count of more than 350 cells per cubic millimeter.
Methods: We randomly assigned HIV-positive adults who had a CD4+ count of more than 500 cells per cubic millimeter to start antiretroviral therapy immediately (immediate-initiation group) or to defer it until the CD4+ count decreased to 350 cells per cubic millimeter or until the development of the acquired immunodeficiency syndrome (AIDS) or another condition that dictated the use of antiretroviral therapy (deferred-initiation group). The primary composite end point was any serious AIDS-related event, serious non-AIDS-related event, or death from any cause.
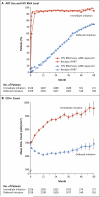
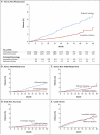
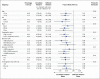
Results: A total of 4685 patients were followed for a mean of 3.0 years. At study entry, the median HIV viral load was 12,759 copies per milliliter, and the median CD4+ count was 651 cells per cubic millimeter. On May 15, 2015, on the basis of an interim analysis, the data and safety monitoring board determined that the study question had been answered and recommended that patients in the deferred-initiation group be offered antiretroviral therapy. The primary end point occurred in 42 patients in the immediate-initiation group (1.8%; 0.60 events per 100 person-years), as compared with 96 patients in the deferred-initiation group (4.1%; 1.38 events per 100 person-years), for a hazard ratio of 0.43 (95% confidence interval [CI], 0.30 to 0.62; P<0.001). Hazard ratios for serious AIDS-related and serious non-AIDS-related events were 0.28 (95% CI, 0.15 to 0.50; P<0.001) and 0.61 (95% CI, 0.38 to 0.97; P=0.04), respectively. More than two thirds of the primary end points (68%) occurred in patients with a CD4+ count of more than 500 cells per cubic millimeter. The risks of a grade 4 event were similar in the two groups, as were the risks of unscheduled hospital admissions.
Conclusions: The initiation of antiretroviral therapy in HIV-positive adults with a CD4+ count of more than 500 cells per cubic millimeter provided net benefits over starting such therapy in patients after the CD4+ count had declined to 350 cells per cubic millimeter. (Funded by the National Institute of Allergy and Infectious Diseases and others; START ClinicalTrials.gov number, NCT00867048.).
Figures
Comment in
-
Overcoming Impediments to Global Implementation of Early Antiretroviral Therapy.N Engl J Med. 2015 Aug 27;373(9):875-6. doi: 10.1056/NEJMe1508527. Epub 2015 Jul 20. N Engl J Med. 2015. PMID: 26193047 No abstract available.
-
Antiretroviral therapy for HIV should be started at diagnosis regardless of CD4+ count, study concludes.BMJ. 2015 Jul 20;351:h3943. doi: 10.1136/bmj.h3943. BMJ. 2015. PMID: 26198644 No abstract available.
-
[HIV-infection: Early therapy is beneficial].Dtsch Med Wochenschr. 2015 Sep;140(18):1338. doi: 10.1055/s-0041-103894. Epub 2015 Sep 11. Dtsch Med Wochenschr. 2015. PMID: 26360940 German. No abstract available.
-
Initiating antiretroviral therapy in HIV-infected patients with >500 CD4 cells/µL provides more benefit than delaying treatment.Evid Based Med. 2016 Feb;21(1):26. doi: 10.1136/ebmed-2015-110307. Epub 2015 Dec 11. Evid Based Med. 2016. PMID: 26660663 No abstract available.
-
In early HIV infection, immediate vs deferred antiretroviral therapy reduced serious illnesses at 3 years.Ann Intern Med. 2015 Dec 15;163(12):JC4. doi: 10.7326/ACPJC-2015-163-12-004. Ann Intern Med. 2015. PMID: 26666806 No abstract available.
-
In HIV-1, immediate vs deferred antiretroviral therapy and isoniazid vs no isoniazid reduced severe illness at 30 mo.Ann Intern Med. 2015 Dec 15;163(12):JC5. doi: 10.7326/ACPJC-2015-163-12-005. Ann Intern Med. 2015. PMID: 26666807 No abstract available.
-
Antiretroviral Therapy in Early HIV Infection.N Engl J Med. 2016 Jan 28;374(4):394. doi: 10.1056/NEJMc1513311. N Engl J Med. 2016. PMID: 26816019 No abstract available.
-
Antiretroviral Therapy in Early HIV Infection.N Engl J Med. 2016 Jan 28;374(4):393. doi: 10.1056/NEJMc1513311. N Engl J Med. 2016. PMID: 26816020 No abstract available.
-
Antiretroviral Therapy in Early HIV Infection.N Engl J Med. 2016 Jan 28;374(4):393-4. doi: 10.1056/NEJMc1513311. N Engl J Med. 2016. PMID: 26816021 No abstract available.
References
-
- Lane HC, Masur H, Gelmann EP, et al. Correlation between immunologic function and clinical subpopulations of patients with the acquired immune deficiency syndrome. Am J Med. 1985;78:417–22. - PubMed
-
- Phillips AN, Lundgren JD. The CD4 lymphocyte count and risk of clinical progression. Curr Opin HIV AIDS. 2006;1:43–9. - PubMed
-
- Lundgren JD, Babiker A, El-Sadr W, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008;197:1145–55. - PubMed
-
- Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–44. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
