Microenvironment interactions and B-cell receptor signaling in Chronic Lymphocytic Leukemia: Implications for disease pathogenesis and treatment
- PMID: 26193078
- PMCID: PMC4715999
- DOI: 10.1016/j.bbamcr.2015.07.009
Microenvironment interactions and B-cell receptor signaling in Chronic Lymphocytic Leukemia: Implications for disease pathogenesis and treatment
Abstract
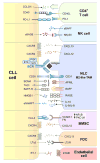
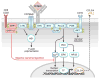
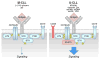
Chronic Lymphocytic Leukemia (CLL) is a malignancy of mature B lymphocytes which are highly dependent on interactions with the tissue microenvironment for their survival and proliferation. Critical components of the microenvironment are monocyte-derived nurselike cells (NLCs), mesenchymal stromal cells, T cells and NK cells, which communicate with CLL cells through a complex network of adhesion molecules, chemokine receptors, tumor necrosis factor (TNF) family members, and soluble factors. (Auto-) antigens and/or autonomous mechanisms activate the B-cell receptor (BCR) and its downstream signaling cascade in secondary lymphatic tissues, playing a central pathogenetic role in CLL. Novel small molecule inhibitors, including the Bruton's tyrosine kinase (BTK) inhibitor ibrutinib and the phosphoinositide-3-kinase delta (PI3Kδ) inhibitor idelalisib, target BCR signaling and have become the most successful new therapeutics in this disease. We here review the cellular and molecular characteristics of CLL cells, and discuss the cellular components and key pathways involved in the cross-talk with their microenvironment. We also highlight the relevant novel treatment strategies, focusing on immunomodulatory agents and BCR signaling inhibitors and how these treatments disrupt CLL-microenvironment interactions. This article is part of a Special Issue entitled: Tumor Microenvironment Regulation of Cancer Cell Survival, Metastasis, Inflammation, and Immune Surveillance edited by Peter Ruvolo and Gregg L. Semenza.
Keywords: BCR; BCR signaling; BCR signaling inhibitors; CLL; Nurselike cells; Stromal cells.
Copyright © 2015 Elsevier B.V. All rights reserved.
Conflict of interest statement
J. A. Burger has received commercial research grants from Pharmacyclics, Gilead, and Portola Pharmaceuticals, and is a consultant/advisory board member for Noxxon, Boehringer Ingelheim Pharma, Janssen, and Pharmacyclics. E. ten Hacken declares no conflicts of interest.
Figures

References
-
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. The New England journal of medicine. 2005;352:804–815. - PubMed
-
- Damle RN, Ghiotto F, Valetto A, Albesiano E, Fais F, Yan XJ, Sison CP, Allen SL, Kolitz J, Schulman P, Vinciguerra VP, Budde P, Frey J, Rai KR, Ferrarini M, Chiorazzi N. B-cell chronic lymphocytic leukemia cells express a surface membrane phenotype of activated, antigen-experienced B lymphocytes. Blood. 2002;99:4087–4093. - PubMed
-
- Klein U, Tu Y, Stolovitzky GA, Mattioli M, Cattoretti G, Husson H, Freedman A, Inghirami G, Cro L, Baldini L, Neri A, Califano A, Dalla-Favera R. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. The Journal of experimental medicine. 2001;194:1625–1638. - PMC - PubMed
-
- Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

