68Ga-Based radiopharmaceuticals: production and application relationship
- PMID: 26193247
- PMCID: PMC6332429
- DOI: 10.3390/molecules200712913
68Ga-Based radiopharmaceuticals: production and application relationship
Abstract
The contribution of 68Ga to the promotion and expansion of clinical research and routine positron emission tomography (PET) for earlier better diagnostics and individualized medicine is considerable. The potential applications of 68Ga-comprising imaging agents include targeted, pre-targeted and non-targeted imaging. This review discusses the key aspects of the production of 68Ga and 68Ga-based radiopharmaceuticals in the light of the impact of regulatory requirements and endpoint pre-clinical and clinical applications.
Keywords: 68Ga; GMP; chemistry; dosimetry; peptide; positron emission tomography; receptor targeting.
Conflict of interest statement
The author declares no conflict of interest.
Figures











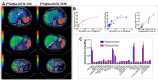

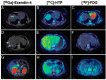
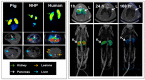



References
-
- Velikyan I. Radionuclides for Imaging and Therapy in Oncology. In: Chen X., Wong S., editors. Cancer Theranostics. Elsevier; Amsterdam, The Netherlands: 2014. pp. 285–325.
-
- Velikyan I. The diversity of 68Ga-based imaging agents. Recent Results Cancer Res. 2013;194:101–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

