Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia
- PMID: 26193301
- PMCID: PMC4517131
- DOI: 10.3390/v7072802
Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia
Abstract
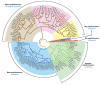
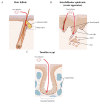
Papillomaviruses have evolved over many millions of years to propagate themselves at specific epithelial niches in a range of different host species. This has led to the great diversity of papillomaviruses that now exist, and to the appearance of distinct strategies for epithelial persistence. Many papillomaviruses minimise the risk of immune clearance by causing chronic asymptomatic infections, accompanied by long-term virion-production with only limited viral gene expression. Such lesions are typical of those caused by Beta HPV types in the general population, with viral activity being suppressed by host immunity. A second strategy requires the evolution of sophisticated immune evasion mechanisms, and allows some HPV types to cause prominent and persistent papillomas, even in immune competent individuals. Some Alphapapillomavirus types have evolved this strategy, including those that cause genital warts in young adults or common warts in children. These strategies reflect broad differences in virus protein function as well as differences in patterns of viral gene expression, with genotype-specific associations underlying the recent introduction of DNA testing, and also the introduction of vaccines to protect against cervical cancer. Interestingly, it appears that cellular environment and the site of infection affect viral pathogenicity by modulating viral gene expression. With the high-risk HPV gene products, changes in E6 and E7 expression are thought to account for the development of neoplasias at the endocervix, the anal and cervical transformation zones, and the tonsilar crypts and other oropharyngeal sites. A detailed analysis of site-specific patterns of gene expression and gene function is now prompted.
Keywords: carcinogenesis; diversity; evolution; human papillomavirus; niche; tissue stem cells; tropism.
Figures





References
-
- PaVE: Papillomavirus Episteme. [(accessed on 11 July 2015)]; Available online: http://pave.niaid.nih.gov/
-
- Bottalico D., Chen Z., Dunne A., Ostoloza J., McKinney S., Sun C., Schlecht N.F., Herrero R., Fatahzadeh M., Schiffman M., et al. The oral cavity contains abundant known and novel human papillomaviruses from the betapapillomavirus and gammapapillomavirus genera. J. Infect. Dis. 2011;204:787–792. doi: 10.1093/infdis/jir383. - DOI - PMC - PubMed
-
- De Koning M.N., Quint K.D., Bruggink S.C., Gussekloo J., Bouwes Bavinck J.N., Quint W.G., Feltkamp M.C., Eekhof J.A. High prevalence of cutaneous warts in elementary school children and the ubiquitous presence of wart-associated human papillomavirus on clinically normal skin. Br. J. Dermatol. 2015;172:196–201. doi: 10.1111/bjd.13216. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

