NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma
- PMID: 26193344
- PMCID: PMC4529359
- DOI: 10.1038/nm.3910
NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma
Abstract
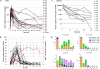
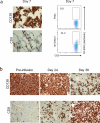
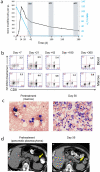
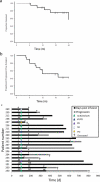
Despite recent therapeutic advances, multiple myeloma (MM) remains largely incurable. Here we report results of a phase I/II trial to evaluate the safety and activity of autologous T cells engineered to express an affinity-enhanced T cell receptor (TCR) recognizing a naturally processed peptide shared by the cancer-testis antigens NY-ESO-1 and LAGE-1. Twenty patients with antigen-positive MM received an average 2.4 × 10(9) engineered T cells 2 d after autologous stem cell transplant. Infusions were well tolerated without clinically apparent cytokine-release syndrome, despite high IL-6 levels. Engineered T cells expanded, persisted, trafficked to marrow and exhibited a cytotoxic phenotype. Persistence of engineered T cells in blood was inversely associated with NY-ESO-1 levels in the marrow. Disease progression was associated with loss of T cell persistence or antigen escape, in accordance with the expected mechanism of action of the transferred T cells. Encouraging clinical responses were observed in 16 of 20 patients (80%) with advanced disease, with a median progression-free survival of 19.1 months. NY-ESO-1-LAGE-1 TCR-engineered T cells were safe, trafficked to marrow and showed extended persistence that correlated with clinical activity against antigen-positive myeloma.
Figures


Comment in
-
Immunotherapy: Engineering a sTrategy for multiple myeloma.Nat Rev Cancer. 2015 Sep;15(9):514. doi: 10.1038/nrc4006. Epub 2015 Aug 20. Nat Rev Cancer. 2015. PMID: 26289313 No abstract available.
References
-
- Tricot G, et al. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87:1196–1198. - PubMed
-
- Alyea E, et al. T-cell--depleted allogeneic bone marrow transplantation followed by donor lymphocyte infusion in patients with multiple myeloma: induction of graft-versus-myeloma effect. Blood. 2001;98:934–939. - PubMed
-
- Lokhorst HM, et al. The occurrence of graft-versus-host disease is the major predictive factor for response to donor lymphocyte infusions in multiple myeloma. Blood. 2004;103:4362–4364. - PubMed
-
- Barlogie B, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89:789–793. - PubMed
-
- Attal M, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. The New England journal of medicine. 1996;335:91–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

