On-Demand Formation of Supported Lipid Membrane Arrays by Trehalose-Assisted Vesicle Delivery for SPR Imaging
- PMID: 26193345
- PMCID: PMC5412510
- DOI: 10.1021/acsami.5b03809
On-Demand Formation of Supported Lipid Membrane Arrays by Trehalose-Assisted Vesicle Delivery for SPR Imaging
Abstract
The fabrication of large-scale, solid-supported lipid bilayer (SLB) arrays has traditionally been an arduous and complex task, primarily due to the need to maintain SLBs within an aqueous environment. In this work, we demonstrate the use of trehalose vitrified phospholipid vesicles that facilitate on-demand generation of microarrays, allowing each element a unique composition, for the label-free and high-throughput analysis of biomolecular interactions by SPR imaging (SPRi). Small, unilamellar vesicles (SUVs) are suspended in trehalose, deposited in a spatially defined manner, with the trehalose vitrifying on either hydrophilic or hydrophobic SPR substrates. SLBs are subsequently spontaneously formed on-demand simply by in situ hydration of the array in the SPR instrument flow cell. The resulting SLBs exhibit high lateral mobility, characteristic of fluidic cellular lipid membranes, and preserve the biological function of embedded cell membrane receptors, as indicated by SPR affinity measurements. Independent fluorescence and SPR imaging studies show that the individual SLBs stay localized at the area of deposition, without any encapsulating matrix, confining coral, or boundaries. The introduced methodology allows individually addressable SLB arrays to be analyzed with excellent label-free sensitivity in a real-time, high-throughput manner. Various protein-ganglioside interactions have been selected as a model system to illustrate discrimination of strong and weak binding responses in SPRi sensorgrams. This methodology has been applied toward generating hybrid bilayer membranes on hydrophobic SPR substrates, demonstrating its versatility toward a range of surfaces and membrane geometries. The stability of the fabricated arrays, over medium to long storage periods, was evaluated and found to be good. The highly efficient and easily scalable nature of the method has the potential to be applied to a variety of label-free sensing platforms requiring lipid membranes for high-throughput analysis of their properties and constituents.
Keywords: HBM; SLB; SPR; SPR imaging; devitrification; microarray.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



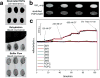

References
-
- Cuatrecasas P. Membrane Receptors. Annu Rev Biochem. 1974;43:169–214. - PubMed
-
- Phillips KS, Cheng Q. Microfluidic Immunoassay for Bacterial Toxins with Supported Phospholipid Bilayer Membranes on Poly-(Dimethylsiloxane) Anal Chem. 2005;77:327–334. - PubMed
-
- Costello DA, Hsia CY, Millet JK, Porri T, Daniel S. Membrane Fusion-Competent Virus-Like Proteoliposomes and Proteinaceous Supported Bilayers Made Directly from Cell Plasma Membranes. Langmuir. 2013;29:6409–6419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

