Regulation of hepatic lipase activity by sphingomyelin in plasma lipoproteins
- PMID: 26193433
- PMCID: PMC4561581
- DOI: 10.1016/j.bbalip.2015.07.003
Regulation of hepatic lipase activity by sphingomyelin in plasma lipoproteins
Abstract
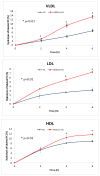
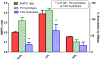
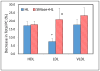
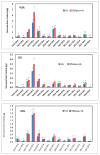
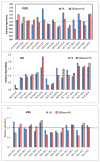
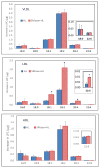
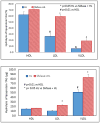
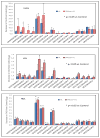
Hepatic lipase (HL) is an important enzyme in the clearance of triacylglycerol (TAG) from the circulation, and has been proposed to have pro-atherogenic as well as anti-atherogenic properties. It hydrolyzes both phospholipids and TAG of lipoproteins, and its activity is negatively correlated with HDL levels. Although it is known that HL acts preferentially on HDL lipids, the basis for this specificity is not known, since it does not require any specific apoprotein for activity. In this study, we tested the hypothesis that sphingomyelin (SM), whose concentration is much higher in VLDL and LDL compared to HDL, is an inhibitor of HL, and that this could explain the lipoprotein specificity of the enzyme. The results presented show that the depletion of SM from normal lipoproteins activated the HL roughly in proportion to their SM content. SM depletion stimulated the hydrolysis of both phosphatidylcholine (PC) and TAG, although the PC hydrolysis was stimulated more. In the native lipoproteins, HL showed specificity for PC species containing polyunsaturated fatty acids at sn-2 position, and produced more unsaturated lyso PC species. The enzyme also showed preferential hydrolysis of certain TAG species over others. SM depletion affected the specificity of the enzyme towards PC and TAG species modestly. These results show that SM is a physiological inhibitor of HL activity in lipoproteins and that the specificity of the enzyme towards HDL is at least partly due to its low SM content.
Keywords: Enzyme inhibition; Hepatic lipase; PC species; Sphingomyelin; Substrate specificity; TAG species.
Published by Elsevier B.V.
Conflict of interest statement
Authors: Peng Yang and P.V. Subbaiah
No conflicts to declare
Figures

References
-
- Zambon A, Deeb S, Pauletto P, Crepaldi G, Brunzell JD. Hepatic lipase: a marker for cardiovascular disease risk and response to therapy. Curr Opin Lipidol. 2003;14:179–189. - PubMed
-
- Zambon A, Bertocco S, Vitturi N, Polentarutti V, Vianello D, Crepaldi G. Relevance of hepatic lipase to the metabolism of triacylglycerol-rich lipoproteins. Biochem Soc Trans. 2003;31:1070–1074. - PubMed
-
- Ehnholm C, Kuusi T. Preparation, characterization, and measurement of hepatic lipase. Methods Enzymol. 1986;129:716–738. - PubMed
-
- Watson TD, Caslake MJ, Freeman DJ, Griffin BA, Hinnie J, Packard CJ, Shepherd J. Determinants of LDL subfraction distribution and concentrations in young normolipidemic subjects. Arterioscler Thromb Vasc Biol. 1994;14:902–910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

