Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients
- PMID: 26193635
- PMCID: PMC4623571
- DOI: 10.1172/JCI81722
Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients
Abstract
Background: Type 1 diabetes (T1D) results from destruction of pancreatic β cells by autoreactive effector T cells. We hypothesized that the immunomodulatory drug alefacept would result in targeted quantitative and qualitative changes in effector T cells and prolonged preservation of endogenous insulin secretion by the remaining β cells in patients with newly diagnosed T1D.
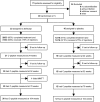
Methods: In a multicenter, randomized, double-blind, placebo-controlled trial, we compared alefacept (two 12-week courses of 15 mg/wk i.m., separated by a 12-week pause) with placebo in patients with recent onset of T1D. Endpoints were assessed at 24 months and included meal-stimulated C-peptide AUC, insulin use, hypoglycemic events, and immunologic responses.
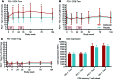
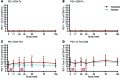
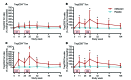
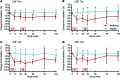
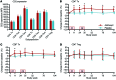
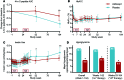
Results: A total of 49 patients were enrolled. At 24 months, or 15 months after the last dose of alefacept, both the 4-hour and the 2-hour C-peptide AUCs were significantly greater in the treatment group than in the control group (P = 0.002 and 0.015, respectively). Exogenous insulin requirements were lower (P = 0.002) and rates of major hypoglycemic events were about 50% reduced (P < 0.001) in the alefacept group compared with placebo at 24 months. There was no apparent between-group difference in glycemic control or adverse events. Alefacept treatment depleted CD4+ and CD8+ central memory T cells (Tcm) and effector memory T cells (Tem) (P < 0.01), preserved Tregs, increased the ratios of Treg to Tem and Tcm (P < 0.01), and increased the percentage of PD-1+CD4+ Tem and Tcm (P < 0.01).
Conclusions: In patients with newly diagnosed T1D, two 12-week courses of alefacept preserved C-peptide secretion, reduced insulin use and hypoglycemic events, and induced favorable immunologic profiles at 24 months, well over 1 year after cessation of therapy.
Trial registration: https://clinicaltrials.gov/ NCT00965458.
Funding: NIH and Astellas.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- TR000006/TR/NCATS NIH HHS/United States
- UM1 AI109565/AI/NIAID NIH HHS/United States
- P30 DK063720/DK/NIDDK NIH HHS/United States
- N01 AI015416/AI/NIAID NIH HHS/United States
- UM2 AI117870/AI/NIAID NIH HHS/United States
- UL1 TR000006/TR/NCATS NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- UM1AI109565/AI/NIAID NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- UL1TR000003/TR/NCATS NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- UL1RR024134/RR/NCRR NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

