Insulin down-regulates the expression of ubiquitin E3 ligases partially by inhibiting the activity and expression of AMP-activated protein kinase in L6 myotubes
- PMID: 26193886
- PMCID: PMC4613693
- DOI: 10.1042/BSR20150017
Insulin down-regulates the expression of ubiquitin E3 ligases partially by inhibiting the activity and expression of AMP-activated protein kinase in L6 myotubes
Abstract
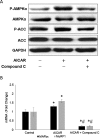
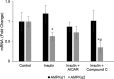
While insulin is an anabolic hormone, AMP-activated protein kinase (AMPK) is not only a key energy regulator, but it can also control substrate metabolism directly by inducing skeletal muscle protein degradation. The hypothesis of the present study was that insulin inhibits AMPK and thus down-regulates the expression of the ubiquitin E3 ligases, muscle atrophy F-box (MAFbx) and muscle RING finger 1 (MuRF1) in skeletal muscle cells. Differentiated L6 myotubes were treated with 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR) and/or compound C to stimulate and/or block AMPK respectively. These treatments were also conducted in the presence or absence of insulin and the cells were analysed by western blot and quantitative real-time PCR. In addition, nucleotide levels were determined using HPLC. The activation of AMPK with AICAR enhanced the mRNA levels of MAFbx and MuRF1. Insulin reduced the phosphorylation and activity AMPK, which was accompanied by reduced MAFbx and MuRF1 mRNA levels. Using a protein kinase B (PKB/Akt) inhibitor, we found that insulin regulates AMPK through the activation of Akt. Furthermore, insulin down-regulated AMPK α2 mRNA. We conclude that insulin inhibits AMPK through Akt phosphorylation in L6 myotubes, which may serve as a possible signalling pathway for the down-regulation of protein degradation. In addition, decreased expression of AMPK α2 may partially participate in inhibiting the activity of AMPK.
Keywords: AMP-activated protein kinase (AMPK); insulin; muscle RING finger 1 (MuRF1); muscle atrophy F-box (MAFbx); myotubes.
© 2015 Authors.
Figures



Similar articles
-
AMP-activated protein kinase enhances the expression of muscle-specific ubiquitin ligases despite its activation of IGF-1/Akt signaling in C2C12 myotubes.J Cell Biochem. 2009 Oct 1;108(2):458-68. doi: 10.1002/jcb.22272. J Cell Biochem. 2009. PMID: 19639604
-
AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells.Am J Physiol Endocrinol Metab. 2007 Jun;292(6):E1555-67. doi: 10.1152/ajpendo.00622.2006. Epub 2007 Jan 30. Am J Physiol Endocrinol Metab. 2007. PMID: 17264220
-
AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes.Biosci Biotechnol Biochem. 2007 Jul;71(7):1650-6. doi: 10.1271/bbb.70057. Epub 2007 Jul 7. Biosci Biotechnol Biochem. 2007. PMID: 17617726
-
Signalling pathways that mediate skeletal muscle hypertrophy and atrophy.Nat Cell Biol. 2003 Feb;5(2):87-90. doi: 10.1038/ncb0203-87. Nat Cell Biol. 2003. PMID: 12563267 Review.
-
PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
Cited by
-
Insulin and Metformin Control Cell Proliferation by Regulating TDG-Mediated DNA Demethylation in Liver and Breast Cancer Cells.Mol Ther Oncolytics. 2020 Jun 24;18:282-294. doi: 10.1016/j.omto.2020.06.010. eCollection 2020 Sep 25. Mol Ther Oncolytics. 2020. PMID: 32728616 Free PMC article.
-
[Roles of adenosine monophosphate activated protein kinase in skeletal muscle atrophy in rats with severe scald].Zhonghua Shao Shang Za Zhi. 2021 Jul 20;37(7):640-646. doi: 10.3760/cma.j.cn501120-20200416-00227. Zhonghua Shao Shang Za Zhi. 2021. PMID: 34304404 Free PMC article. Chinese.
-
High glucose alters tendon homeostasis through downregulation of the AMPK/Egr1 pathway.Sci Rep. 2017 Mar 7;7:44199. doi: 10.1038/srep44199. Sci Rep. 2017. PMID: 28266660 Free PMC article.
-
Update on the synergistic effect of HSL and insulin in the treatment of metabolic disorders.Ther Adv Endocrinol Metab. 2019 Sep 20;10:2042018819877300. doi: 10.1177/2042018819877300. eCollection 2019. Ther Adv Endocrinol Metab. 2019. PMID: 31565213 Free PMC article. Review.
-
A novel stable isotope tracer method to simultaneously quantify skeletal muscle protein synthesis and breakdown.Metabol Open. 2020 Mar;5:100022. doi: 10.1016/j.metop.2020.100022. Metabol Open. 2020. PMID: 32494771 Free PMC article.
References
-
- Coppack S.W., Jensen M.D., Miles J.M. In vivo regulation of lipolysis in humans. J. Lipid. Res. 1994;35:177–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

