TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG)
- PMID: 26194186
- PMCID: PMC4507314
- DOI: 10.1186/s12885-015-1529-x
TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG)
Abstract
Background: The optimal management of patients with resectable gastric cancer continues to evolve in Western countries. Following publication of the US Intergroup 0116 and UK Medical Research Council MAGIC trials, there are now two standards of care for adjuvant therapy in resectable gastric cancer, at least in the Western world: postoperative chemoradiotherapy and perioperative epirubicin/cisplatin/fluorouracil (ECF) chemotherapy. We hypothesize that adding chemoradiation to standard perioperative ECF chemotherapy will achieve further survival gains. We also believe there are advantages to administering chemoradiation in the preoperative rather than postoperative setting. In this article, we describe the TOPGEAR trial, which is a randomised phase III trial comparing control arm therapy of perioperative ECF chemotherapy with experimental arm therapy of preoperative chemoradiation plus perioperative ECF chemotherapy.
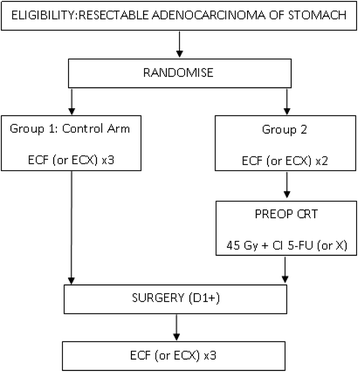
Methods/design: Eligible patients with resectable adenocarcinoma of the stomach or gastroesophageal junction will be randomized to receive either perioperative chemotherapy alone (3 preoperative and 3 postoperative cycles of ECF) or perioperative chemotherapy plus preoperative chemoradiation. In the chemoradiation arm, patients receive 2 cycles of ECF plus chemoradiation prior to surgery, and then following surgery 3 further cycles of ECF are given. The trial is being conducted in two Parts; Part 1 (phase II component) has recruited 120 patients with the aim of assessing feasibility, safety and preliminary efficacy of preoperative chemoradiation. Part 2 (phase III component) will recruit a further 632 patients to provide a total sample size of 752 patients. The primary endpoint of the phase III trial is overall survival. The trial includes quality of life and biological substudies, as well as a health economic evaluation. In addition, the trial incorporates a rigorous quality assurance program that includes real time central review of radiotherapy plans and central review of surgical technique.
Discussion: TOPGEAR is an international, intergroup collaboration led by the Australasian Gastro-Intestinal Trials Group (AGITG), in collaboration with the Trans-Tasman Radiation Oncology Group (TROG), European Organisation for Research and Treatment of Cancer (EORTC) and the NCIC Clinical Trials Group. It addresses a globally significant question that will help inform future international standards for clinical practice in resectable gastric cancer.
Trial registration: Australian New Zealand Clinical Trials Registry: ACTRN12609000035224 . Registered 30 May 2009.
Figures
References
-
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. - DOI - PubMed
-
- Ajani JA, Mansfield PF, Janjan N, Morris J, Pisters PW, Lynch PM, Feig B, Myerson R, Nivers R, Cohen DS, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol. 2004;22:2774–2780. doi: 10.1200/JCO.2004.01.015. - DOI - PubMed
-
- Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, Greskovich JF, Anne PR, Bradley JD, Willett C, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953–3958. doi: 10.1200/JCO.2006.06.4840. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical