Impact of the Alzheimer's Disease Neuroimaging Initiative, 2004 to 2014
- PMID: 26194320
- PMCID: PMC4659407
- DOI: 10.1016/j.jalz.2015.04.005
Impact of the Alzheimer's Disease Neuroimaging Initiative, 2004 to 2014
Abstract
Introduction: The Alzheimer's Disease Neuroimaging Initiative (ADNI) was established in 2004 to facilitate the development of effective treatments for Alzheimer's disease (AD) by validating biomarkers for AD clinical trials.
Methods: We searched for ADNI publications using established methods.
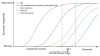
Results: ADNI has (1) developed standardized biomarkers for use in clinical trial subject selection and as surrogate outcome measures; (2) standardized protocols for use across multiple centers; (3) initiated worldwide ADNI; (4) inspired initiatives investigating traumatic brain injury and post-traumatic stress disorder in military populations, and depression, respectively, as an AD risk factor; (5) acted as a data-sharing model; (6) generated data used in over 600 publications, leading to the identification of novel AD risk alleles, and an understanding of the relationship between biomarkers and AD progression; and (7) inspired other public-private partnerships developing biomarkers for Parkinson's disease and multiple sclerosis.
Discussion: ADNI has made myriad impacts in its first decade. A competitive renewal of the project in 2015 would see the use of newly developed tau imaging ligands, and the continued development of recruitment strategies and outcome measures for clinical trials.
Keywords: AD biomarker signature; Alzheimer's disease; Amyloid phenotyping; Clinical trial biomarkers; Data-sharing; Tau imaging; Worldwide ADNI.
Copyright © 2015 The Alzheimer's Association. All rights reserved.
Figures
References
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. - PubMed
-
- Khachaturian ZS. Toward a comprehensive theory of Alzheimer’s disease—challenges, caveats, and parameters. Ann N Y Acad Sci. 2000;924:184–93. - PubMed
-
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. - PubMed
-
- Petersen RC, Waring SC, Smith GE, Tangalos EG, Thibodeau SN. Predictive value of APOE genotyping in incipient Alzheimer’s disease. Ann N Y Acad Sci. 1996;802:58–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical