Analysis of the interplay of protein biogenesis factors at the ribosome exit site reveals new role for NAC
- PMID: 26195668
- PMCID: PMC4508901
- DOI: 10.1083/jcb.201410086
Analysis of the interplay of protein biogenesis factors at the ribosome exit site reveals new role for NAC
Abstract
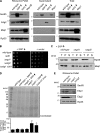
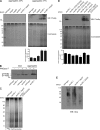
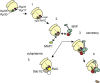
The ribosome exit site is a focal point for the interaction of protein-biogenesis factors that guide the fate of nascent polypeptides. These factors include chaperones such as NAC, N-terminal-modifying enzymes like Methionine aminopeptidase (MetAP), and the signal recognition particle (SRP), which targets secretory and membrane proteins to the ER. These factors potentially compete with one another in the short time-window when the nascent chain first emerges at the exit site, suggesting a need for regulation. Here, we show that MetAP contacts the ribosome at the universal adaptor site where it is adjacent to the α subunit of NAC. SRP is also known to contact the ribosome at this site. In the absence of NAC, MetAP and SRP antagonize each other, indicating a novel role for NAC in regulating the access of MetAP and SRP to the ribosome. NAC also functions in SRP-dependent targeting and helps to protect substrates from aggregation before translocation.
© 2015 Nyathi and Pool.
Figures




Comment in
-
Translation: competition at the ribosome exit site.Nat Rev Mol Cell Biol. 2015 Sep;16(9):516. doi: 10.1038/nrm4046. Epub 2015 Aug 12. Nat Rev Mol Cell Biol. 2015. PMID: 26265407 No abstract available.
References
-
- Becker T., Bhushan S., Jarasch A., Armache J.P., Funes S., Jossinet F., Gumbart J., Mielke T., Berninghausen O., Schulten K., et al. . 2009. Structure of monomeric yeast and mammalian Sec61 complexes interacting with the translating ribosome. Science. 326:1369–1373. 10.1126/science.1178535 - DOI - PMC - PubMed
-
- Boissel J.P., Kasper T.J., and Bunn H.F.. 1988. Cotranslational amino-terminal processing of cytosolic proteins. Cell-free expression of site-directed mutants of human hemoglobin. J. Biol. Chem. 263:8443–8449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

