Influences of clonality on plant sexual reproduction
- PMID: 26195747
- PMCID: PMC4517233
- DOI: 10.1073/pnas.1501712112
Influences of clonality on plant sexual reproduction
Abstract
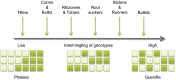
Flowering plants possess an unrivaled diversity of mechanisms for achieving sexual and asexual reproduction, often simultaneously. The commonest type of asexual reproduction is clonal growth (vegetative propagation) in which parental genotypes (genets) produce vegetative modules (ramets) that are capable of independent growth, reproduction, and often dispersal. Clonal growth leads to an expansion in the size of genets and increased fitness because large floral displays increase fertility and opportunities for outcrossing. Moreover, the clonal dispersal of vegetative propagules can assist "mate finding," particularly in aquatic plants. However, there are ecological circumstances in which functional antagonism between sexual and asexual reproductive modes can negatively affect the fitness of clonal plants. Populations of heterostylous and dioecious species have a small number of mating groups (two or three), which should occur at equal frequency in equilibrium populations. Extensive clonal growth and vegetative dispersal can disrupt the functioning of these sexual polymorphisms, resulting in biased morph ratios and populations with a single mating group, with consequences for fertility and mating. In populations in which clonal propagation predominates, mutations reducing fertility may lead to sexual dysfunction and even the loss of sex. Recent evidence suggests that somatic mutations can play a significant role in influencing fitness in clonal plants and may also help explain the occurrence of genetic diversity in sterile clonal populations. Highly polymorphic genetic markers offer outstanding opportunities for gaining novel insights into functional interactions between sexual and clonal reproduction in flowering plants.
Keywords: clonal growth; dioecy; geitonogamy; heterostyly; somatic mutations.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
-
- Richards AJ. Plant Breeding Systems. Chapman and Hall; London, UK: 1997.
-
- Klimes L, Klimesov J, Hendriks R, van Groenendael JM, de Kroon H. 1997. Clonal plant architecture: A comparative analysis of form and function. The Ecology and Evolution of Clonal Plants, eds de Kroon H, van Groenendael (Backhuys, Leiden, The Netherlands), pp 1–29.
-
- Sculthorpe CD. The Biology of Aquatic Vascular Plants. Edward Arnold; London: 1967.
-
- Maynard Smith J. The Evolution of Sex. Cambridge Univ Press; Cambridge, UK: 1978.
-
- Vallejo-Marín M, Dorken ME, Barrett SCH. The ecological and evolutionary consequences of clonality for plant mating. Annu Rev Ecol Evol Syst. 2010;41:193–213.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

